You can also download the Book of Abstracts in PDF format.
Last updated on 26 August, 2009.
INVITED SPEAKERS
O1
Free electron transfer . relations between molecule dynamics and reaction kinetics
O. Brede, S. Naumov, University of Leipzig, Wilhelm Ostwald Institute of Physical and Theoretical Chemistry,
Leibniz Institute for Surface Modification, Leipzig, Germany
The electron transfer in non-polar media (alkanes, alkyl chlorides) exhibits some essential peculiarities. For instance the reaction of hetero-substituted aromatics with parent solvent radical cations results in the parallel formation of metastable donor radical cations and fragmentation products, in comparable amounts. The fragmentation products originate from a dissociative donor radical cation which decays extremely rapidly, i.e., in some femtoseconds. This phenomenon is explained in terms of intramolecular dynamic motions which cause changes of the electron density (π- and n-orbitals) in dependence on the deformation angle between the substituents and the aromatic ring. Hence femtosecond dynamics is reflected in the nanosecond time range and can be observed with real-time spectroscopy. Therefore, the process is named free electron transfer (FET) which corresponds to an unhindered electron jump occurring in the first approach of the reactants. From the FET mechanism some new aspects for chemical reaction kinetics can be derived.
O2
Hydrated electron absorption in high temperature water: is oscillator strength conserved?
D. Bartels, Notre Dame Radiation Laboratory, Notre Dame, Indiana, USA
The intense absorption spectrum of the hydrated electron, with maximum at 720 nm in room temperature water, shifts strongly to the red as the temperature is raised. In order to use this strong signal for dosimetry and calculation of second order reaction rates, the extinction coefficient must be measured as a function of temperature (and also pressure in supercritical water).
Determination of the absolute extinction coefficient of a transient is a difficult undertaking. We have used two pulse radiolysis methods. In the first, we directly observe the kinetics of hydrated electron scavenging by methyl viologen, to form the strongly colored MV+ cation. The extinction coefficient of this long-lived radical was carefully measured by electrochemical and temperature cycling many years ago, so the measured ratio of hydrated electron absorption to MV+ absorption gives the desired extinction coefficient of (e-)aq. The MV+ spectrum has only been quantitatively measured up to 200 oC, so the method is not valid at higher temperature. In the second method, transient absorption of hydrated electron is recorded as it is being scavenged by N2O or SF6. Direct comparison of the transient absorption with the measured (N2 or F-) product yield allows the extinction coefficient to be calculated.
While the purpose of our study was to investigate high temperature water, we were astonished to discover that the room temperature hydrated electron extinction coefficient has been incorrectly reported (low) by 10%. We can now demonstrate how this error arose in calibration of pulse radiolysis yields. It has been remarked for decades that the integrated oscillator strength of the hydrated electron is less than unity. Our new measurements rectify this problem for room temperature, and we will present results for high temperature water.
O3
The nature of holes in carbon doped titania
J. Rabani, Accelerator Laboratory at The Hebrew University of Jerusalem, Jerusalem 91904, Israel
It is well known that semiconductors (SC) produce conduction band electrons and valence band holes upon band gap excitation. The mobile species become quickly trapped at the surface.
The most popular semiconductor is titanium dioxide, where the reactive surface holes (hT+) have been recently identified as surface .O·. (or .·OH depending on pH) covalently linked to Ti atoms. Most organic compounds are oxidized by the holes. These holes react similarly to ·OH radicals and hence there is some resemblance between the photochemistry of TiO2 and radiolysis, although in the case of TiO2 the reactions take place on the surface.
Titanium dioxide has many favorable properties for application as a photocatalyst for decontamination of water from organic materials, but is lacking absorption in the visible range, where photons are relatively cheap. In addition the quantum yield of reaction with solutes in water is too low under conditions required by industrial water treatment due to the competition between electron-hole recombination and localization at the surface. The discovery that doping of TiO2 leads to extension of the photoactive region from UV to visible light has remarkably increased the interest in such doped TiO2, and a large number of materials have been developed on the basis of this strategy. We.ll focus on carbon doped TiO2 where the visible photoactivity is attributed to introduction of intragap localized carbon states or organic segments. Visible photolysis of aerated carbon doped TiO2 (C-TiO2) aqueous suspensions induces oxidation of the model compound used, namely methanol. The effects of absorbed light density, added hydrogen peroxide and added catalase on the rate of HCHO formation have been studied. The mechanism has been shown to involve oxidation of CH3OH by surface trapped holes, although these holes have lower energy than those formed upon UV photolysis of undoped TiO2. The C-TiO2 electrons reduce O2 to H2O2. UV photolysis of the aerated aqueous suspensions of C-TiO2 (KRONOS vlp 7000) or TiO2 (KRONOS uvlp 7500, Degussa P25) containing CH3OH produces both HCHO and H2O2.
Comparison between UV and visible excitation of C-TiO2 and between C-TiO2 and undoped TiO2reveals qualitative differences with respect to the nature of the oxidizing and reducing species. The carbon doping decreases the yield both in visible and UV excitation compared to the undoped material (UV). Prospects for the future application of doped TiO2 for photocatalysis will be discussed.
O4
Competition between ion recombination and scavenging
N. Green, A. Agarwal, Department of Chemistry, University of Oxford, UK
In low permittivity solvents ion recombination is dominated by the effects of the relative drift of the ions caused by the Coulomb attraction. However, such systems are frequently investigated by scavenging methods. Since the work of Tachiya on electric field effects, drift has been known to affect the steady-state scavenging rate constant. However, during the recombination drift depends on the instantaneous distance between the ions, and is therefore inherently transient.
This paper describes an investigation of this problem using simulation methods. It is found that, within the constraint of the diffusion approximation, there are conditions where the Smoluchowski time-dependent rate constant underestimates the degree to which scavenging intercepts geminate recombination. For this to be a substantial effect the initial distance between the ions must be relatively small (e.g. 4 nm) compared to the typical thermalisation distance of an electron (e.g. 8 nm).
Simulations have been used to generate numerical time-dependent rate constants for scavenging. But these proved barely more successful than the Smoluchowski theory, in spite of having been calculated from the simulation results.
Stratification of results by recombination time shows that there is a strong correlation between the recombination time and the scavenging time. It was hypothesised that this correlation arises through the strong transient drift as the ions approach one another. This hypothesis was confirmed by the application of a novel simulation method in which the ion trajectories are simulated conditional on the recombination time. It was found that in every case the scavenging rate increases sharply just prior to recombination.
This dependence of scavenging rate on recombination time is a fundamental breakdown of the assumptions underlying both the theory of diffusion kinetics and the IRT method. Nonetheless, a path decomposition method has been devised that allows IRT simulations to be corrected for this effect with good accuracy.
O5
Scavenging of es- and OH. radicals in concentrated acidic aqueous solutions, a new mechanism for reactivity of UV in acidic solution
M. Mostafavi, Laboratoire de Chimie Physique/ ELYSE /UMR 8000
CNRS . Université Paris-Sud, Bât 349, 91405 Orsay, France
The direct ionization of aqueous solutions by highly energetic photons or accelerated particles leads to the decomposition of the water molecule and results in the ultrafast formation of highly reactive radicals that are initially non-homogeneously distributed in the solution along the irradiation track. The determination of the initial radiolytic yields of the formation of these primary species, and their evolution during the spur expansion is the center of interest of many studies in the field of radiation chemistry. The ability to predict the evolution of systems exposed to ionizing radiation is important to a wide field of applications: nuclear power plants and waste treatment and storage, radiotherapy and radiobiology, electron beam depolution, and others. During the 1980s and 1990s, a wealth of pulse radiolysis and radical scavenging measurements were performed to obtain the time dependent radiolytic yield of different free radicals and molecular products. Recently, the values for the yield of the solvated electron were re-evaluated for water. Nevertheless, the initial G value of the OH. radical is still controversial. In neutral and acidic aqueous solutions containing 2 mol L−1 of Cl−, we studied the decay of the solvated electron and the formation of ClOH.- and Cl2.- by picosecond pulse radiolysis experiment. We estimated the yield of the OH. radical at 100 ps to be 5.0 ×10−7 mol J−1.
In the framework of our studies on spent nuclear fuel it is also necessary to have a better understanding of the radiolytic transformation of the fourth valency of uranium, UIV. In fact, the spent nuclear fuel or waste are supposed to be buried deep underground or in salt mines. Several problems still remain to be resolved and one of the aspects studied is to consider a possible incident where the container fails by corrosion or the salt mines are exposed to water. In these cases UIV can be oxidized to very soluble hexavalent uranium, UVI, which promotes radionuclide release to the biosphere. In this particular case, it is also important to have a realistic understanding of the mechanism of the radiolytic processes involved in the complex medium. Thanks to the radiolytic yields measurements, we present a new mechanism for oxidation of UIV in acidic solution.
O6
Stochastic treatment of exciton decay dynamics in quantum dots and nanotubes
M. Tachiya, National Institution of Advanced Industrial Science and Technology (AIST),
Tsukuba, Ibaraki 305-8565, Japan
Exciton decay dynamics in semiconductor quantum dots and single-walled carbon nanotubes are extensively studied experimentally [1]. Excitons produced in these nanoobjects decay either by radiative process or by charge transfer to ligands bound to them [1a,1b] or by Auger recombination [1c,1d]. The number of excitons produced in each nanoobject is usually very small and fluctuations in the number of excitons in each nanoobject are comparable to the average number of excitons per nanoobject. In this situation the conventional bulk approach that considers only the average number of excitons per nanoobject is not sufficient to describe the dynamics of exciton decay. Instead one has to use a stochastic approach which properly takes into account fluctuations in the number of excitons in each nanoobject. In this talk I present a stochastic treatment of exciton decay dynamics in nanoobjects and analyse recent experiments on exciton decay in quantum dots by charge transfer to ligands [2], exciton decay in quantum dots and nanotubes by Auger recombination [3, 4], and competition between charge transfer to ligands and Auger recombination in exciton decay in quantum dots [5]. The quantized character of the number of excitons in each nanoobject turns out to be essential to understand exciton decay dynamics.
References:
[1] See, for example, (a) A. Boulesbaa et al. (b) V. V. Matylitsky et al.: J. Am. Chem. Soc. 131, 2424 (2009); (c) V. I. Klimov et al.: Science 287, 1011 (2000); (d) F. Wang et al: Phys. Rev. B 70, 241403(R) (2004)
[2] S. Sadhu, A. Patra, M. Tachiya: to be published
[3] A. V. Barzykin, M. Tachiya: Phys. Rev. B 72, 075425 (2005)
[4] A. V. Barzykin, M. Tachiya: J. Phys. Condens. Matter 19, 065105 (2007)
[5] M. Hilczer, M. Tachiya: to be published
O7
Reactions of radicals at surfaces: radicals on powders and nano-particles in aqueous suspension
D. Meyerstein, Biological Chemistry Department, Ariel university Center of Samaria,
Ariel Israel and Chemistry Department, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Radicals are present at solid-solution interfaces of metals, semi-conductors and insulating oxides in a variety of processes, e.g.:
1. Catalitic processes
2. Electrochemical reactions
3. Photochemical processes in which the light is absorbed by solid
Consequently, the mechanisms of reaction of radicals with surfaces are of utmost importance. Recent results show that alkyl radicals and alkyl-peroxyl radicals react in aqueous solutions with suspended metal- and semiconductor- surfaces to form intermediates with metal-carbon or metal-oxygen bonds, respectively. The results suggest that the life time of these intermediates is considerably longer than expected. The mechanisms of decomposition of these intermediates depend on the nature of the metal, substituents on R, pH, temperature, and electrical potential bias.
O8
IAEA role in radiation technology support for advanced materials development and cleaner industrial processes
M. Haji-Saeid, A. Sáfrány, M. H. O. Sampa, N. Ramamoorthy, International Atomic Energy Agency,
Wagramer Strasse 5, P.B. 100, A-1400, Vienna, Austria
The IAEA is implementing projects on applications of radiation technology in the major areas aimed to support capacity building in developing Member States. Besides the well established application of radiation for sterilization of various products, radiation technology has shown promise for treatment of organic pollutants in flue gases, wastewaters and sludge, and is also found effective for preservation of precious objects of art affected by fungi and insects. The preparation of new, value added-materials/products is made possible through radiation treatment of low-value materials, waste agricultural by-products and natural polymers. Nanoscale engineering of materials is a rapidly developing area of various radiation techniques. Besides the synthesis and modification, radiation techniques are widely used for investigation of the reaction mechanisms as well as for the characterization of such new materials and products.
The role of IAEA as the only UN organization dealing with radiation technology in fostering international cooperation is crucial in all the above areas due to the multi-disciplinary expertise required in these fields. Coordinated Research Projects (CRP) and Technical Cooperation (TC) projects are the two major channels used to support radiation technology adaptation and building national capabilities in developing MS. Additionally, consultancy and technical meetings are organized on selected subjects, and international meetings, conferences and workshops are being supported. All these activities have been found valuable for deriving benefits in the healthcare, agriculture, research and industrial sectors in developing Member States.
This lecture will highlight the IAEA activities in radiation technology together with some success stories on the transfer of these technologies to the developing Member States of the IAEA.
O9
Cleaning water by AOPs: an update
S. Emmi, B. Esposito, M. Saracino, ISOF-CNR and ENVIREN, Via P. Gobetti, 101, 40129 Bologna,
E. Ferri, SMETEC Dept., UNIBO, Via S. Donato, 15, 40127 Bologna, Italy
Advancements in analytical sciences for environmental monitoring and protection should nowadays be followed by efforts in the investigation and optimization of remediation processes. Advanced Oxidation Processes (AOPs) have demonstrated their effectiveness and efficiency in transforming organic pollutants in non-harmful compounds. Investigating AOPs means to describe the hydroxyl radical induced mineralization of the organic substrates found to pollute water. In the AOPs, H2O itself - and eventually H2O2 - is split to produce OH by injecting energetic electrons, gamma rays, UV light or ultrasounds in water1,2. A series of reactions take place in atmospheric aqueous solutions as p bond addition, hydrogen abstraction, electron transfer, and peroxydation which progressively demolish the organic pollutants up to elementary molecules like CO2 and H2O.
An AOP system aims to mimic the naturally occurring oxidation:
CH2O + O2 . CO2 + H2O (1)
where CH2O is a symbolic organic compound.
The equivalent Advanced Oxidation Process can be schematically conceived as
CH2O + 2OH . CO2 + H2O + H2 (2)
In this work emphasis is given to the latest results on the decomposition of surfactants in water.
References:
1 S. S Emmi, E. Takács: Water Remediation by E-beam method, in .Radiation Chemistry - From basics to applications in material and life sciences., EDP Sciences, ISBN 978-2-7598-0024-7, 2008, Ch. 6, 79
2. H. Suty; C. De Traversay, M. Cost: Water Sci.Tech. 49, 227 (2004)
O10
Wastewater treatment with electron beam
B. Han, J. K. Kim, Y. R. Kim, EB TECH Co., Ltd., Daejeon, Republic of Korea
N. Zommer, Pele Inc., Milpidas Ca. USA
Advanced wastewater treatment is essential in the effective treatment of municipal and industrial wastewater to protect public health and to meet water quality criteria for aquatic environment, water recycling and reuse. Electron beam treatment of wastewater leads to purification by the decomposition of pollutants as a result of their reactions with highly reactive species formed from water radiolysis: hydrated electron e-aq, OH free radical and H atom. Sometimes such reactions are accompanied by the other processes, and the synergistic effect upon the use of combined methods such as electron beam with biological treatment, adsorption and others improves the effect of electron beam treatment of the wastewater purification.
Pilot scale researches on industrial wastewater, contaminated underground water and effluent from municipal wastewater plant by electron beam are carried out in several countries. First industrial scale plants were constructed in Voronezh, Russia for reduction of chemicals in underground water, and after that, commercial scale plant with 400 kW electron accelerator has constructed for treating 10,000 m3/day of textile dyeing wastewater in Daegu, Korea. Cost assessments were carried out for this plant, and it showed the operation cost was not more than USD 1 M per year and it was about USD 0.3 per each m3 of wastewater.
The key to the successful implementation of electron beam process in environmental protection depends on how to manage the economics in its application. To compete with other processes in economic evaluation, the electron beam plant should be operated with cost-effective manners. To result in complete decomposition of the pollutants, sufficiently high absorbed doses are required. However, in real conditions of rather high content of pollutants in wastewater, high absorbed doses are not economically acceptable, and it is better to utilize the partial decomposition of pollutant as well as transformations of pollutant molecules that result in improving subsequent purification stages.
O11
Radiation damage: from amino acids to proteins
I. Carmichael, Radiation Laboratory, University of Notre Dame, Notre Dame, IN 46556, USA
E. F. Garman, Laboratory of Molecular Biophysics, Dept. of Biochemistry, University of Oxford,
South Parks Road, Oxford, OX1 3QU, UK
We discuss various aspects of radiation damage to proteins and their constituent amino acids.
Topics covered include radiolytic oxidative degradation of solitary amino acids where a complete description of radical production is presented for some of the simplest cases. Predictions from ab initio molecular orbital calculations and Density Functional Theory are shown to be validated by concurrent1,2 and new experimental observations, particularly from time-resolved EPR spectroscopy.
Radiation damage continues to plague efforts to collect high quality data during X-ray macromolecular crystallography at synchrotrons even at cryotemperatures (around 100 K),3 where an experimental dose limit (30 MGy) has been established beyond which satisfactory data will not be collected.4 Both general diffraction pattern degradation and specific structural damage to particular amino acid residues clearly call out for a radiation chemical explanation. In pursuit of this goal, room temperature experiments have uncovered a surprising inverse dose rate effect on the value of D1/2 (the dose which causes the diffraction intensity to become half its original value).5 We show how the addition of specific radical scavengers can strongly modify the observed functional form of the dose dependence of the intensity loss during room temperature data collection.6
References:
1. Hug, Carmichael, Fessenden: J. Chem. Soc., Perkin Trans. 2, 907 (2000)
2. Wisniowski, Carmichael, Fessenden, Hug: J. Phys. Chem. A. 106, 4573 (2002)
3. Ravelli, Garman: Curr. Opin. Struct. Biol. 16, 624 (2006)
4. Owen, Rudi.o-Pi.era, Garman: Proc. Nat. Acad. Soc. 103, 4912 (2006)
5. Southworth-Davies, Medina, Carmichael, Garman: Structure 15, 1531 (2007)
6. Barker et al.: J. Synchr. Radiat. 16, 205 (2009)
O12
Tautomerism in the guanyl radical and related systems
C. Chatgilialoglu, ISOF, Consiglio Nazionale delle Ricerche, 40129 Bologna, Italy
The main reaction of the HO. radical with guanosine or 2.-deoxyguanosine (~65%) is not the addition at the C4 position as previously thought, but the hydrogen abstraction from the NH2 moiety to give a guanyl radical. This radical, characterized by a broad band in the visible region (around 610 nm), undergoes a water-assisted tautomerization to the most stable isomer, which is also known as one-electron oxidation species of the corresponding guanine derivatives. Moreover, one-electron reduction of 8-bromoguanine derivatives was found to give a radical anion that undergoes fast protonation at C8 by proton transfer from the solvent to afford a complex. This complex debrominates giving two short-lived intermediates, which are recognized to be the two-guanyl tautomers. The tautomerization is a general phenomenon of purine reactive intermediates that has been uncovered so far, and will add other important information to the general scenario of the radical transformations concerning DNA.
References:
1. Angew. Chem. Int. Ed. 44, 6030 (2005)
2. J. Am. Chem. Soc. 128, 13796 (2006)
3. J. Phys. Chem. B 112, 5209 (2008)
4. J. Phys. Chem. B 113, 2170 (2009)
5. Angew. Chem. Int. Ed. 48, 2214 (2009)
O13
Radiation-induced damage to DNA-protein complexes
M. Spotheim-Maurizot, S. Goffinont, D. Genest, Centre de Biophysique Moléculaire, CNRS,
rue Charles Sadron, 45071 Orléans, France
M. Davidkova, Department of Radiation Dosimetry, Nuclear Physics Institute, 18086 Prague 8,
Czech Republic
A key step in the regulation of gene expression, DNA structuring and DNA repair is the binding of some proteins to specific DNA sequences. When such complexes are irradiated, the DNA-binding proteins are acting as efficient protectors against the attack of hydroxyl radicals produced by water radiolysis. They protect their binding sites on DNA by shielding and by radicals scavenging. They also modify the conformation of DNA (compaction, bending) thereby rendering DNA more resistant to radiolysis. But proteins are also vulnerable and get damaged under irradiation. The progressive accumulation of damages on the protein (mainly side chain modifications) induces the loss of its ability to bind to the specific DNA sequence.
In the case of low dose irradiation of the E. coli lactose operator-repressor complex, the protein is still bound to DNA and protects its specific binding site. With increasing dose, the protein starts to get damaged: a change of its oligomerisation state (from tetramer to dimer) occurs in parallel with the damage of the DNA-binding domains (headpieces). At high doses, the DNA-tetramer and the DNA-dimer complexes are entirely disrupted.
CD data show that upon irradiation, the structure and the stability of the headpieces changes. Mass spectrometry data complemented by RADACK calculations allow identifying all the oxidised amino-acids of the headpieces (mainly Tyr converted to DOPA). Molecular dynamics simulations reveal and characterize the structural changes induced by Tyr oxidation in the headpiece. Moreover they show a loss of stability and binding energy as well as changes in the structure of the complex between DNA and oxidized headpieces with respect to the native complex.
The change of the oligomerisation state can be explained by the oxidation of the amino-acids of the tetramerisation domain, predicted by RADACK calculations (mainly Leu).
In conclusion, irradiation affects the properties of the protein, and consequently hinders their binding to DNA, due to the oxidation of critical amino-acids. The biological consequence can be the disturbance of the processes in which the DNA-protein complexes are involved (gene regulation, DNA repair, etc.).
O14
Radiation induced reactions involving sulfur-centered radicals
K. Bobrowski, Institute of Nuclear Chemistry and Technology, 03-195 Warszawa, Poland
Sulfur-centered radicals represent an important class of radicals since they exhibit very interesting redox chemistry, including biological redox processes, and different spectral and kinetic properties as compared to carbon-centered radicals. This is due to the fact that the lone electron pairs present in sulfur atom influenced on the overall electronic structure of radicals.
In the past few years unprecedented progress has been made in the recognition and understanding the role of structures and reaction mechanisms of sulfur-centered radicals. Research on these transients flourished paricularly in systems that are relevant in biology, biochemistry and medicine. Relevant examples will highlight only the very recent achievements emerging from radiation chemical studies. These include radical processes connected with the intramolecular addition of cysteine thiyl radicals (CysS·) to phenylalanine, reversible H-atom transfer between CysS· and amino acids within a peptide chain, addition reaction of thiyl radicals (RS·) involving double bonds in pyrimidines, RS·-catalyzed cis/trans isomerization of lipid double bonds in unsaturated fatty acids, ·OH and ·H-induced degradation of molecules containing sulfur atoms, stabilization of monomeric sulfur radical cations (>S+·) with peptide bonds, and stabilization of disulfide radical anions (>S\S<) in uracil-derived disulfides.
Important outputs from these studies are new directions for improving our knowledge how sulfur-centered radicals interact with major cellular tragets during oxidative stress, i.e. proteins, DNA and lipids.
O15
Application of radiation for synthesis, modification and sterilization of polymeric biomaterials
J. Dzierzawska, S. Kadlubowski, A. Kaszubska, J. Komasa, A. K. Olejnik, B. Rokita, K. Sobczyk, P. Ulanski,
R. A. Wach, J. M. Rosiak, Institute of Applied Radiation Chemistry, Technical University of Łodz,
Wroblewskiego 15, 93-590 Łodz, Poland
D. Grijpma, Institute for Biomedical Technology (BMTI) and Department of Polymer Chemistry and Biomaterials, Faculty of Science and Technology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands
P. Lenas, Department of Biochemistry and Molecular Biology IV, University of Complutense, Madrid, Spain
One of the greatest challenges for polymer chemistry and engineering is to devise materials suitable for medical applications, including tissue engineering products and other implants. An indispensable step in the manufacturing of polymeric biomaterials is sterilization. Application of radiation technique for this purpose has many advantages, but the potential negative side effects, as degradation and post-irradiation reactions, have to be taken into account. In this talk, irradiation effects on some typical biodegradable polymers [e.g., poly(caprolactone, various forms of poly(lactic acid) and poly(trimethylene carbonate)] will be discussed, with particular stress on the influence of irradiation on the application-related properties.
Radiation can be used also for synthesizing biomaterials for tissue engineering. At our laboratory, we have applied radiation polymerization and crosslinking to obtain hydrogel scaffolds for three-dimensional neural tissue culture. The challenges of finding the optimum chemical composition and physical form of this material will be discussed.
Finally, a brief review will be presented on the application of ionizing radiation for development of thermosensitive polymers to be used as substrates for tissue culture.
This work has been financed in part by the European Commission (VI FP Projects .Artemis., .Protec. and .Custom-IMD.), Ministry of Science and Higher Education, Poland (SPB grant 503/6.PR UE/2007/7, 138/E-370/SPB/IAEA/KN /DWM 77/2005-2007 and 507/6.PR UE/2008/7) as well as European Regional Development Fund, project UDA-POIG.01.03.01-00-088/08-00.
O16
Radio-fluorogenic co-polymerization: a new (chemical) method of dose monitoring and imaging
J. M. Warman, M. P. de Haas, L. H. Luthjens, Delft University of Technology, The Netherlands
We have developed a new type of radiation dosimeter which is based on radio-fluorogenic co-polymerization .RFCP.. In this method the dosimeter consists of a liquid monomer which polymerizes when exposed to high energy radiation, for example methylmethacrylate. Dissolved in this bulk monomer is a small (ca millimolar) concentration of a compound that is non-fluorescent but which becomes fluorescent when it is incorporated into a growing polymer chain. The resulting fluorescence is then proportional to the yield of initiating free-radicals formed and hence to the accumulated radiation dose. The method is illustrated by application to remote, real-time dose monitoring within a small (< 0.2 ml) volume. Using a semi-rigid gel form of the RFCP dosimeter, two and three-dimensional fluorescent images of a masked 3 MeV electron beam and a 6 MeV X-ray beam have been created.
O17
Charge transport in dynamic DNA
Y. Berlin, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113, USA
Migration of excess charges through DNA has been a focus of considerable interest. So far most theoretical works were restricted to the description of charge motion along the well-defined static DNA structure [1]. The two important parameters that determine the mechanism of charge transfer in DNA are site energies and charge transfer integrals. Both parameters critically depend on geometric fluctuations in DNA [2].
I describe the magnitude of fluctuations in energies and charge transfer integrals along the donor-DNA-acceptor system. The consequences of fluctuations are discussed using stilbene-capped DNA hairpins [3]. We exploited molecular dynamics simulations and density functional theory calculations to study the time-scale of fluctuations in the site energies and charge transfer integrals. The results were used in tight-binding calculations to evaluate the effects of structural fluctuations on hole transfer in the DNA hairpins. The injection energy barrier is higher than the average values of the charge transfer integrals. The fluctuations in site energies and charge transfer integrals are sufficiently large to lead to the domination of a fluctuation-assisted incoherent transport. The difference between the present and previous explanations is the lack of a priori assumptions about a change in the transport mechanism (from superexchange to hopping) typical for earlier works [4]. In contrast with earlier studies, the proposed model of charge transport in fluctuating (rather than static) DNA structure provides a theoretical framework to describe the process for all bridge lengths. The results are in agreement with the full range of experimental data.
References:
1. Y. A. Berlin et al.: DNA electron transfer processes: Some theoretical notions. Long-Range
ChargeTransfer in DNA, ed. G. Schuster, Springer (Heidelberg, 2004)
2. K. Senthilkumar et al.: J. Am. Chem. Soc. 127, 14894 (2005)
3. F. D. Lewis et al.: Angew. Chem. Int. Ed. Eng. 45, 7982 (2006)
4. Y. A. Berlin, A. L. Burin, M. A. Ratner. Chem. Phys. 275, 61 (2002)
O18
Reactivity of oxyiron(VI, V, and VI) with inorganic and organic compounds in aqueous solution: pulse radiolysis studies
V. K. Sharma, R. A. Yngard, Chemistry Department, Florida Institute of Technology, Melbourne, Florida 32901,
D. Cabelli, Chemistry Department, Brookhaven National Laboratory, Long Island, NY 11973, USA
The involvement of high-valent iron-oxo intermediates in biological, environmental, and industrial processes is of current interest. FeVIO42- is relatively stable and is a potential oxidant in .green. treatment of polluted waters. By contrast, Fe(V) and Fe(IV) are short-lived transients when produced in aqueous solution in the absence of strongly bonding ligands other than hydroxide, a feature that has limited studies of its reactivity. FeVO43- and FeIVO44- have been suggested to be the intermediates in the oxidation of inorganic and organic compounds by FeVIO43-. FeVO43- can be generated easily in the presence of excess FeVIO42- through the use of reducing carbon-centered radicals produced in pulse radiolysis. The decay of the oxyiron(V) species is dependent on pH, where completely deprotonated FeVO43- decays to a longer lived transient (t1/2 ≈ seconds) via a first-order process. However, as the pH is lowered, oxyiron(V) disappears by second-order kinetics to form ferric ions and hydrogen peroxide. The second-order rate constant observed in the disappearance of Fe(V) increases as the pH is lowered and is of the order of 107 M-1s-1. Oxyiron(IV) complex with a simple inorganic ligand, P2O74- in basic medium can be generated from the corresponding parent complex by oxidation with OH/O- radical in aqueous solutions. The pyrophosphate complex of iron(IV), formed at pH ≥ 10 is short lived (t1/2 = 100-600 ms). This complex of iron(IV) disappears by a second-order process to form a Fe(III) pyrophosphate complex and molecular oxygen.
A premix pulse radiolysis was used to measure the reactivity of FeVIO42-, FeVO43-, and FeIVO44- with cyanides, oxysulfur species, and aminopolycarboxylates (APCs) and their radicals. The oxidation rates decrease with increase in pH and are, in the order of FeVO43- > FeIVO44- > FeVIO42-. Reduction rate constants of FeVIO42- to FeVO43- by ·CONH2, ·SO3- and S4O6·3- radicals were found to be 2.6 ± 0.6x109, 1.9 ± 0.3x108 and 7.5 ± 0.8 x 107 M-1 s-1. The oxidation of cyanides by Fe(V) indicate a two-electron transfer with SCN- while one-electron reduction of FeV to FeIV to FeIII takes place in case of CN-. The formation of a Fe(IV)-cyano complex in reduction of FeV by metal-cyanide will be discussed. The reaction rates of FeVO43- with the two oxysulfur ions were separated by an order of magnitude, with SO32- reacting at 3.9 ± 0.3x104 while S2O32- reacted with Fe(V) at 2.1±0.1x103 M-1 s-1 at pH 11.4. Measurements for FeVO43- reactivity with oxy-sulfur species suggest two-electron reduction. Fe(V) reactivity with APCs at pH 12.5 increase in order tertiary < secondary < primary amines and proceed via a two-electron oxidation.
O19
Quantitative and multiscale assessment of network structure in UV- and EB-polymerized multiacrylates
X. Coqueret, M. Krzeminski, ICMR,CNRS UMR 622, Université de Reims Champagne Ardenne,
BP 1039, 51687 Reims Cedex
M. Molinari, M. Troyon, LMEN, EA 379, Université de Reims Champagne Ardenne,
BP 1039, 51687 Reims Cedex, France
The radiation-induced polymerization of multiacrylates is known to generate microheterogeneous networks. In order to gain an insight into the polymer microstructure, a combination of analytic methods was used to quantify polymer segment mobility in the different domains. Solid state proton T2 NMR relaxation experiments were performed on radiation-cured materials prepared from model difunctional monomers. This method allowed us to distinguish two phases inside the materials: one consisting in rigid domains, and a second one with higher local mobility and distinct relaxation features. The decay of transverse magnetization is fitted with two components, (short or long T2), which can be assigned to the highly cross-linked and the loosely cross-linked phase, respectively. The influence of acrylate conversion on the relaxation behavior of cured samples was examined to describe the gradual evolution of the different domains, in terms of local mobility and associated fraction of material, as the radiation-induced polymerization proceeds. AFM analysis of the networks in the phase imaging mode provides a complementary picture of the network with indications on the actual dimensions of the soft and rigid domains. MDSC thermograms can be further analyzed in the light of these results. Comparing the NMR relaxation data as well as the calorimetric features of networks prepared by UV- or by EB-induced polymerization does not reveal noticeable differences to be related to the initiation mechanism and/or curing conditions. Various structural and kinetic data will be discussed for interpreting the observed polymerization behavior of the model diacrylate.
O20
The synthesis of polymer nano hydogels using pulsed electron beams
M. Al-Sheikhly, J. Silverman, Materials Science and Engineering Department,
University of Maryland, College Park, MD, USA
Nano-hydrogels made of bio-compatible hydrophilic polymers can be used in various medical applications such as drug delivery and imaging. Intravenously introduced hydrogel-drug conjugate (10 - 200 nm particles can be effectively accumulated in tissues/organs by prolonged circulation and can be selectively transported into tumor tissues by the EPR (enhanced permeability and retention) effect. We are investigating the radiation-induced synthesis of functionalized polymer nano-hydrogels that can serve as targeted nano-medicine carriers. The latest results on the synthesis and kinetic analysis of poly(vinyl pyrrolidone) (PVP) nanogels in dilute aqueous solutions using γ-rays and electron beams, particularly at high temperature, will be presented. At temperatures above 60 °C, PVP chains start to collapse decreasing its average hydrodynamic radius, Rh from 23 (at 20 °C) to 15.6 nm (at 80 °C) due to the disruption of polymer-water hydrogen bonds. The collapsed form of the PVP molecules enhances the intra-crosslinking reactions of the radiolytically produced free radicals leading to a further decrease in its average Rh to the value of 14 nm (γ-ray irradiation with 10 kGy). The nano-gel structure was also synthesized using pulsed electron beam irradiation at high repetition rates, which give rise to a high intra-chain yield of multiple free radicals. These free radicals enhance the intra-crosslinking reactions leading to the formation of smaller size nanogel molecules with average Rh value of 12 nm at 300 pulses per second. At high pulse repetition rates, the intra-molecular crosslinking reactions of the carbon centered free radicals are preferred; this effect is enhanced at higher temperatures. While the high dose rate pulses enhance the intra-molecular crosslinking, low dose rate pulses and the extended shape of the PVP molecules favor inter-molecular crosslinking. From the pulse radiolysis, the second order reaction rate constants (2k) of PVP radical recombination are determined are 1.1 . 2.8 x 109 L mol-1 s-1 [(PVP-H)●, ε390 nm = 510 ± 30 L mol-1 cm-1] The activation energy (Ea) of this reaction is calculated from the Arrhenius plot of PVP radical decay rate constants at a series of temperatures and have a value of ca. 1.0 (below 60 °C) and 6.8 kcal mol-1 (above 60 °C). Two Ea can be explained by the different rate-determining mechanisms of PVP radical recombination reaction at the two temperature regions. Low Ea (at below 60 °C) reflects the diffusion controlled polymer radical reaction in good solvent. But higher temperature above 60 °C, polymer chains are segregated from the aqueous solution by micro-phase separation and collapse. The movement of the polymer radical chain is limited largely to within the region in which it is almost solid in character and so Ea for radical recombination at high temperature is higher than that obtained for lower temperature. The Rh of radiolytically produced hydrogel nano-particles were measured by means of the dynamic light scattering (DLS) experiments.
YOUNG CAREER SCIENTISTS
Y1
Water Radiolysis with Heavy Ions
S. Yamashita, Advanced Science Research Center, Japan Atomic Energy Agency (ASRC, JAEA), Ibaraki, Japan
Y. Katsumura, Dept. Nucl. Engin.Management, Sch. Engin., Univ. Tokyo, Tokyo, Japan / ASRC, JAEA
T. Maeyama, Dept. Nucl. Engin.Management, Sch. Engin., Univ. Tokyo, Tokyo, Japan
M. Lin, Advanced Science Research Center, Japan Atomic Energy Agency (ASRC, JAEA), Ibaraki, Japan
Y. Muroya, Nucl. Prof. School, Sch. Engin., Univ. Tokyo, Japan
T. Murakami, Res. Center for Chrareged Part. Therapy, National Institute of Radiological Sciences, Japan
J. Meesungnoen, J.-P. Jay-Gerin, Fac. de Méd. Sciences de la Sante Univ. de Sherbrooke, Québec, Canada
Water radiolysis with high-energy heavy ions has been investigated through measurement of product yield and simulation of intra-track reactions. Ions from helium to xenon of energies up to 500 MeV/u were taken for irradiation at the biological irradiation port of HIMAC installed at NIRS, Japan. Taking long range of the ions, typically longer than 10 cm, as an advantage, track-segment yields of main water decomposition products, e-aq, .OH and H2O2, have been determined. Utilizing energy absorber made of PMMA, ion energies were decreased down to about 10 MeV/u to vary beam properties in sample solutions, and then, product yields for wide ranges of ion types and energies were accumulated [1-2]. Influences of scavenger concentration have also been discussed and yield of approximate sum of .OH, H. and e-aq was measured [3]. In parallel to these measurements, Monte-Carlo simulation of intra-track reactions has been conducted not only to reproduce the experimental results but also to discuss further track structure and its dynamics from microscopic viewpoint.
References:
[1] S. Yamashita et al.: Radiat. Phys. Chem. 77, 439 (2008)
[2] S. Yamashita et al.: Radiat. Phys. Chem. 77, 1224 (2008)
[3] S. Yamashita et al.: Radiat. Res. 170, 521 (2008)
Y2
Redox reactions of cerium complexes in aqueous solutions
G. Yardeni, I. Zilbermann, E. Maimon, Chemistry Department, Nuclear Research Centre Negev, Beer-Sheva, and Chemistry Department, Ben-Gurion University of the Negev, Beer-Sheva, Israel
L. Kats, Chemistry Department, Nuclear Research Center Negev, Beer-Sheva, Israel
H. Cohen, D. Meyerstein, Ben-Gurion Univ. of the Negev, Beer-Sheva and Biological Chemistry Dept., Ariel University Center of Samaria, Ariel, Israel
Cerium is the largest lanthanide, and it is considered a hard acid. Several studies in the past have shown that CeIII reacts with aminocarboxylate ligands, e.g. EDTA. Catecholeamine type ligands are known as stabilizers of CeIV, lowering the redox potential of the CeIV/III couple. 5,7-Dioxo-1,4,8,11-tetraazacyclotetradecane ("dioxocyclam"), L1, is a macrocyclic ligand which has two amido groups and two secondary amines. Macrocyclic dioxotetraamines are known as stabilizers of high valence transition metal ions. It is of interest to investigate stabilization of CeIV by dioxocyclam, even though the latter is a relatively large cation.
Previously it was shown [1] that L1 reacts with CeIII to give a complex that was characterized by UV-Vis spectroscopy, and was oxidized by persulphate anions and by few inorganic radicals. Recently [2] it has been proved that this CeIII-L1 complex reacts with the organic methyl radical to give an intermediate with a Ce-C bond. It has been also found that this cerium complex is oxidized by molecular oxygen.
Recent results also point out, that other hard bases like pyrophosphate and adenosine triphosphate (ATP) stabilize high valent lanthanide and transition metal ions [3,4]. Thus it has been of interest to check whether analogous ligands . tri(poly)phosphate and cyclic triphosphate ions stabilize high valent lanthanides as well. The complexes of CeIII with tri(poly)phosphate (L2) and cyclic triphosphate (L3) were synthesized, and their reactions with organic and inorganic radicals were investigated.
Detailed kinetics and reaction mechanism for these reactions will be presented.
References:
[1] G. Yardeni et al.: 24th Miller Conference on Radiation Chemistry (2005)
[2] G. Yardeni et al.: Research on Chemical Intermediates, (2009), in press
[3] D. Shamir et al.: Eur. J. Inorg. Chem., 523 (2006)
[4] G. Yardeni et al.: to be published
Y3
Peculiarities of proton transfer reaction of sterically hindered amine radical cations studied by optically detected EPR in irradiated solutions
M. M. Vyushkova, V. A. Bagryansky, Yu. N. Molin, Institute of Chemical Kinetics and Combustion,
Novosibirsk, Russia
Sterically hindered piperidine type amines are used as light stabilizers and precursors of stable nitroxide radicals. The peculiarities of their structure are reflected in the reactivity of corresponding radical cations (RC.s). Sterically hindered amine RC.s are believed to play the key role in antioxidant action of their parent amines as well as in the photoinitiated reactions of selective ω-monooxidation of aliphatic organic substrates by N-haloamines. In this work, the peculiarities of proton transfer between sterically hindered amines and their RCs have been studied by optically detected EPR method. OD EPR technique detects selectively the particles possessing both spin and electrical charge. The method is sensitive to low concentrations of radiation-generated radical ions in liquids. In comparison with ordinary EPR techniques, these advantages of OD EPR facilitate detection of the processes accompanied by separation of spin and charge, such as RC proton transfer reaction.
We have used the following amine compounds, listed in the order of decreasing steric crowding around the transferring proton: di-tert-amylamine (DTA), 2,2,6,6-tetramethylpiperidine (TMP), 1,2,2,6,6-pentamethylpiperidine (PMP), 2,6-dimethylpiperidine, piperidine. Triethylamine (TEA) has been employed as reference compound.
TMP RC has proved to undergo incomplete proton transfer yielding dimeric complex radical cation (I). In contrast, the RC of DTA, an acyclic analogue of TMP, has displayed no changes at our timescale. PMP RC has proved liable to transfer β-proton though the reaction is considerably slower than in TEA. As for piperidine and 2,6-dimethylpiperidine RC.s, they seem to decay so fast that they fall out of OD EPR time domain (ns).
Thus, given specific degree of steric crowding of the reactive site (as for TMP), steric effect of bulky substituents results in stabilization of the distonic intermediates. The effect is sensitive to local environment of the transferring proton. The technique allowed the first detection and unambiguous identification of the intermediate complex.
The financial support of RFBR (Grant No 08-03-00741) is greatly acknowledged.
Y4
Radiation-induced transformations of acetylenic hydrocarbons in solid noble gas matrices
A. V. Kobzarenko, I. A. Baranova, I. V. Tyulpina, V. I. Feldman, Department of Chemistry,
M.V. Lomonosov Moscow State University, Moscow, 119991 Russia
The photo- and radiation chemistry of acetylene in solid noble gas matrices has attracted interest in connection with synthesis of novel-type, unusual high-energy organic molecules and radicals, such as HNgCCH (Ng = Xe, Kr), HXeCCXeH, and HXeCC.1, 2, 3 A common way of preparation of such species involves dissociation of acetylene in a noble-gas matrix below 20 K followed by anneling-induced mobilization of H atoms. Quantum chemical calculations also predict stability of xenon derivatives of higher acetylenes (or even oligomers). However the mechanistic aspects of the radiation-chemical transformations are unclear. In this report we present the results of matrix-isolation studies of radiation-induced transformations of propyne and tert-butyl acetylene (3,3-dimethylpropyne-1) in solid Xe and Kr matices using a combination of EPR and IR spectroscopy. The deposited matrix samples (ca. 1:1000) were irradiated with X-rays at 15 - 17 K and annealed at 30 - 32 K (krypton) or 40 . 45 K (in xenon). In the case of propyne, the dissociation of (C≡C).H bond is quite efficient, however, the resulting radical·C≡CCH3 appears to be unstable at 16 K. In the case of t-butyl acetylene, we found EPR evidences for formation of methyl radicals, H atoms and R·CH2 type radicals originating from t-butyl group. The IR data reveal formation of methane directly after irradiation at 16 K. C.C bond rupture increases when turning from Xe to Kr matrix, which may be explained by effect of excess energy in positive hole or excitation transfer. We were unable to detect the products of Xe or Kr insertion to acetylenic moiety. We can conclude that alkyl substituent has crucial effect on the mechanism of radiation-induced transformations of acetylene derivatives in solid noble-gas matrices. Pathways and prospects of synthesis of organo-noble-gas compounds from various alkynes are discussed.
The work was supported by INTAS (no 05-1000008-8017) and Russian Foundation for Basic Research (no. 09-03-00848).
References:
1. L. Khriachtchev et al: J. Am. Chem. Soc. 125, 4696 (2003)
2. V. I. Feldman, F. F. Sukhov, A. Y. Orlov, I. V. Tyulpina: J. Am. Chem. Soc. 125, 4698 (2003)
3. L. Khriachtchev et al.: J. Am. Chem. Soc. 125, 6876 (2003)
Y5
Synthesis of novel stimuli responsive copolymer containing NIPAAm and APMA onto PP films by one step γ-ray irradiation
A. Contreras-García, E. Bucio, Departamento de Química de Radiaciones y Radioquímica, Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510 México, D. F. Mexico
C. Alvarez-Lorenzo, A. Concheiro, Departamento de Farmacia y Tecnología Farmacéutica, Universidad de Santiago de Compostela, 15782-Santiago de Compostela, Spain.
Grafting may be carried out pre-irradiating the substrate or irradiating the substrate in the presence of the monomers. Currently, a great variety of stimuli-responsive copolymers suitable for biomedical applications, including drug delivery, has been synthesized. In this work a polypropylene (PP) films was grafted with N-isopropylacrylamide (NIPAAm) and N-(3-aminopropyl) methacrylamide (APMA) in order to achieve pH- and temperature-responsiveness. APMA has been used in medical devices owing to its hemocompatibility. The grafted film was obtained by oxidative pre-irradiation method in one step using a gamma source of 60Co. The optimal conditions as reaction time, monomers concentrations and doses were searched to get grafting percents suitable for subsequent studies in biomedical applications. The characterization of the graft polymer obtained was carried out with FTIR-ATR, TGA, DSC and water contact angle measurements. The degree of swelling was also evaluated to characterize the responsiveness of the grafted films to temperature and pH. NIPAAm exhibits a low critical solution temperature (LCST) close to 32°C, but when this monomer was copolymerized with other hydrophilic monomer, the LCST increased and transition range became wider.
This work was supported by DGAPA-UNAM (Grant No. IN200208), Mexico, and by MICINN and FEDER (SAF2008-01679), and the Xunta de Galicia (PGIDT07CSA002203PR), Spain.
Y6
Novel analytical technique to study nucleobase influence on DNA strand breaks caused by direct ionizing radiation
R. M. Watson, W. A. Bernhard, University of Rochester School of Medicine and Dentistry, Rochester NY, USA
Analysis of the reactions involved in the direct effect of ionizing radiation on DNA is crucial to assessing the risks related with exposure at low dose. The direct interaction of ionizing radiation with DNA initially results in free radicals situated on bases and the backbone, which eventually lead to stable end products that include strand breaks (sb) and free nucleobase release (fbr). The yields of these two products are thought to be related because ejection of an electron from the DNA backbone produces a radical cation that deprotonates to yield a neutral carbon-centered deoxyribose radical. These neutral radicals react when dissolved to produce one strand break and one free base each. Therefore fbr can be used as an indicator of sb.
It is commonly presumed that that sb occur independent of the surrounding base context. However recent studies have indicated that a base may indeed have influence over the probability of sb at its backbone unit. In one such study, films prepared from 10- to 30-mer DNA duplexes were irradiated at RT under air using X-rays generated by a tungsten tube operated at 70 kV. The films were dissolved in nuclease free water and stored at 277 K. Unaltered free base release was measured using HPLC, and the yields determined for each base were not strictly proportionate to their presence in the DNA sequence. In fact, this study indicated that strand breaks may be influenced by a number of factors including position within the oligomer as well as the base and its base context.
The current study involves further analysis of these factors; instead of using HPLC to separate and measure fbr, which is time consuming and expensive, a novel analytical technique is being used to determine the amount and ratio of fbr for each of the four bases. This technique involves separation of free bases from bulk DNA using filters followed by decomposition of the UV spectra of mixtures of bases at different pH. Decomposition is performed using the Levenberg.Marquardt algorithm for non-linear optimization. To date we have been able to determine relative amounts of nucleobase in mixtures of all four bases with accuracy comparable to HPLC while using much smaller quantities of DNA.
Supported by PHS Grant 2-R01-CA32546 of the NCI.
Y7
Release of nitrite from the antitubercular bioreductive drug PA-824 and analogs upon one-electron reduction in protic, low dielectric medium
A. Maroz, S. Shinde, W. A. Denny, B. D. Palmer, R. F. Anderson, Departments of Chemistry and Auckland Cancer Society Research Centre, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand
The bicyclic nitroimidazoles PA-824 and OPC-67683 are new candidate drugs for treating TB, and are in clinical trial. The drugs appear to act in an atypical manner compared to related nitroimidazoles, which are generally reduced by cellular reductases to the nitro radical anion, which either undergoes redox cycling to form superoxide (oxic conditions), or leads to the formation of nitroso- and on to hydroxylamine cytotoxins (under hypoxia). The major metabolites of PA-824 and OPC-67683 determined under aerobic and hypoxic conditions respectively, are their des-nitro compounds. Recently it has been reported that these drugs release the reactive nitrogen species (RNS) NO when metabolized in the bacterium.1 To explore possible mechanisms by which RNS can be released, we have studied a series of nitroimidazoles related to PA-824, using pulse and steady-state radiolysis. Propan-2-ol medium (neat and with addition of 5% vol. water) has been chosen as a model for a solvent-restricting active site.
In contrast to results obtained in aqueous solution, where reduction of the imidazole ring preceeds the reduction of the nitro group,2 we observed the release of nitrite upon the steady-state radiolytic reduction of PA-824 and analogs, with yields up to approximately one-electron equivalent. The yield of nitrite release was dependent on the electronic properties of the 3-substituent attached to the imidazole ring which forms part of the 6-membered saturated ring of PA-824. The analogs with SO and SO2 as substituents did not yield nitrite upon one-electron reduction, and CH2, S substituents produced low yields of nitrite. Changing the saturated 6-member ring to a 7-membered ring, also containing O as the substituent, inhibited the production of nitrite. Parallel experiments for PA-824 in 95/5% propan-2-ol/water, did not change the nitrite yield. MS analysis of the des-nitro product, formed on radiolytic reduction, gave an m/e mass increase over that of the parent compound of +13. This product is assigned as the adduct between 2-propanol and the desnitro compound and could result from radical-radical reaction.
Pulse radiolysis was used to produce the transient spectrum arising from one-electron reduction in 95/5% vol. propan-2-ol/water. The spectrum is similar to that of related nitroimidazoles in aqueous solutions. Spectra in deaerated and N2O saturated solutions exhibit differences in 280 nm region; in deaerated solution dose independent formation of the des-nitro radical of the parent compound at 6000 s-1 was observed. The absorption band centred at 450 nm, typical of one-electron reduced nitroimidazoles, was observed to decay with 1st-order kinetics at 700 s-1, independent of both the absorbed dose and nitroimidazole concentration.
References:
1. R. Singh, U. Manjunatha, H. I. M. Boshoff, Z. H. Ha: Science, 322, 1392 (2008)
2. R. F. Anderson et al.: Org. Biomol. Chem., 6, 1973 (2008)
Y8
In silico study of cellulose radicals produced by gamma irradiation
I. Stanculescu, Department of Physical Chemistry, Faculty of Chemistry, University of Bucharest,
4-12 Bd. Regina Elisabeta, 030018 Bucharest, Romania
M. Cutrubinis, D. C. Negut, I. V. Moise, IRASM Irradiation Technology Center, .Horia Hulubei. National Institute for Physics and Nuclear Engineering, 407 Atomistilor Str., 077125, Magurele, Ilfov County, Romania
A paper characteristic is the appearence of cellulose free radicals. A single signal C (gsymm = 2,004) may be observed in the EPR spectra of non irradiated paper. In the case of irradiated paper, the intensity of the signal C becomes usualy greater and, in addition, a pair of lines occurs to the left (at lower field) and right (at higher field) of the central signal. This pair of lines is due to cellulose free radicals formed by the ionizing radiation. The spacing of this radiation-induced signal pair is about 6,05 mT and is symptomatic of radiation treatment having taken place. Gamma irradiation leads to the formation of carbon centered radicals. For cellulose, 5 different types of radicals produced by H atom elimination from a C-H bond were analysed using the AM1 and PM3 semi-empirical quantum mechanics methods. Final radiation chemical changes of cellulose are consequences of unimolecular or bimolecular reactions of the radicals. Enthalpy change for the reaction schemes of the C1 radical: (I) b-cleavage followed by opening of the anhydroglucose ring or breaking of the glucoside bond and formation of carbonyl groups and (II) radical scavenging by oxygen or water was calculated and the most probable reaction mechanism was assessed.
POSTERS
P1
Influence of UV and gamma irradiaton on wave propagation in the Belousov-Zhabotinsky reaction
S. Castillo-Rojas, Departamento de Química de Radiaciones y Radioquímica, Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, 04510 México D.F.
J. Ignés-Mullol, F. Sagués, Departament de Química Física, Universitat de Barcelona, Diagonal 647, E-08028, Barcelona, Spain
The aim of this work was to compare BZ reaction without irradiation and irradiating with ultraviolet radiation and γ rays from a 60Co source. The idea was to work with a system quasi-2D layered. The sample was prepared from sodium silicate gels of 0.5 cm thickness and 14 cm diameter, in which ferroin catalyst was fixed. The other compounds of BZ reaction: NaBrO3 + malonic acid + NaBr in sulphuric acid were irradiated. We determined the velocities and number of wave-fronts generated during 2 hours reaction. We compared both irradiated systems through the intermediate HBrO2 which is produced by photolysis and radiolysis. Some implications about reaction mechanisms with experimental data obtained, are discussed.
P2
Energy deposition by low energy electron beams in polymer materials
I. Enomoto, Tokyo Metropolitan Industrial Technology Research Institute: The University of Tokyo
Y. Katsumura, The University of Tokyo: Advanced Science Research Center, Japan Atomic Energy Agency
H. Kudo, The University of Tokyo, Japan
Recently in the field of radiation processing, application of low energy electron beam having energy of 200 keV or less becomes popular for the surface treatment of the material without damage of the substrate. In fact, new low energy electron beam accelerators have been introduced to the market in Japan. However, the understanding of the behavior of low electrons for the practical application seems not sufficeient, because the low electrons are easily influenced by scattering and absorption. In the present experiment, in order to visualize the transportation of low energy electrons, a Monte Carlo calculation code named EGS5 (electron gamma shower) has been taken and the clculated dose distribution have compared with the depth profiles of radical formation measured by ESR.
It is found that the calculated dose profile to the electron irradiation of 200 keV is in good agreement with the radical distribution as a function of depth determined by ESR. From the calculation for the irradiation with 100 keV electrons, energy losses at titanium (Ti) foil as a window and nitrogen (N2) gas thickness between the window and target material are significant and not negligible. For example, 60% of energy is deposited in the 10 μm-thick Ti foil and 30 mm N2 gas layer, and finally only about 20% of the initial energy can be absorbed by polymer films. When the thickness of Ti foil was reduced by half, the energy deposition in the sample became doubled. On the contrary, even if the thickness of N2 gas layer is changed, almost no change for the energy deposition in the sample was found. These differences are related to the density of Ti foil and N2 gas layer. Therefore, much care should be taken for the selection of the thickness of the Ti foil.
Although new dosimeter films to measure the dose distribution at the surface of the material are highly expected to be developed, it is concluded that the calculation with EGS5 is inevitable and essential.
P3
Fine tuning in radiation chemistry: model studies on bridged bifunctional radical ions
V. I. Feldman, K. B. Nuzhdin, A. V. Kobzarenko, I. A. Baranova, D. A. Tyurin, Department of Chemistry,
Moscow State University, Moscow, 119991 Russia
Bridged bifunctional molecules of general structure X-(CH2)n-Y represent an interesting class of models for experimental and theoretical investigations of the early stages of radiation-induced processes in complex organic molecules and macromolecules, including those of biological importance. Variations in ionization energy of functional groups (X and Y), bridge length and conformation may affect crucially the electronic structure and reactivity of primary ionized molecules, which can be described in terms of .fine tuning.. This contribution gives an overview of our recent studies on a number of bifunctional radical cations (in particular, diketones amidoesters, amidoamines and aminoethers) using EPR, optical spectroscopy and quantum-chemical calculations [1 . 3]. The criterion of localization of spin density in bridged radical cations will be formulated. The effect of .magic bridge. (or .conformational lock.) on the reactivity of radical cations will be considered in detail. Several examples of selective and specific reactions for radical cations with a .magic bridge. (n = 3) will be presented. In addition, preliminary data on the properties of bridged bifunctional radical anions will be discussed.
This work was supported by the Russian Foundation for Basic Research (project no. 09-03-00848).
References:
[1] K. B. Nuzhdin, V. I. Feldman, A. V. Kobzarenko: J. Phys. Chem. A, 11, 13294 (2007)
[2] K. B. Nuzhdin, V. I. Feldman: Radiat. Phys. Chem., 77, 416 (2008)
[3] K. B. Nuzhdin, A. V. Kobzarenko, I. I. Barabanov, V. I. Feldman: Mendeleev Commun., 2009, in press.
P4
Reaction kinetics in supercritical fluids probed by muon spin spectroscopy
K. Ghandi, P. Cormier, C. Alcorn, Mount Allison University, Sackville, New Brunswick, Canada
P. W. Percival, J.-C. Brodovitch, Simon Fraser University, Burnaby, British Columbia, Canada
Muonium (Mu = μ+e−) is, from the chemical point of view, simply a light isotopic analogue of the H-atom. We have recently extended Mu chemical kinetics to supercritical fluids which are medium of interest for a variety of chemical applications from green chemistry to future generation nuclear reactors (Gen IV reactors). Fundamental investigation of chemical reactions requires the detection and monitoring of intermediates, often transient free radicals, which are difficult to detect under the extremes of pressure and temperature that define near critical and supercritical fluids. Many types of Mu reactions have been studied in sub- and supercritical water and CO2, and Mu kinetics have been used to deduce the nature of H atom chemical dynamics under extreme conditions that are very difficult to study by other techniques. Unlike the optical detection methods usually employed in pulse radiolysis studies, muon spin spectroscopy is sensitive only to the transient under study (Mu in this case), and is unaffected by environmental effects (scattering of light, change in extinction coefficient, etc.). This presentation will review our measurements of kinetics in water and CO2 under sub- and supercritical conditions.
P5
Yield of OH near the Bragg peak of heavy-ion beam from HIMAC
T. Maeyama, Y. Muroya, School of Engineering, University of Tokyo
S. Yamashita, Advanced Science Research Center, JAEA
G. Baldacchino, Saclay, Commissariat . l'Énergie Atomique, France
Y. Katsumura, School of Engineering, University of Tokyo,Advanced Science Research Center, JAEA
M. Taguchi, A. Kimura, Quantum Beam Science Directorate, JAEA
T. Murakami, National Institute of Radiological Science (NIRS), Japan
Aqueous solution of Coumarin-3-carboxylic acid (CCA) has been applied to yield measurement of .OH in water radiolysis with carbon ion beams. Production yield of a fluorescent probe, 7OH-CCA, which is a stable product produced after scavenging reaction for .OH by CCA, was determined by using HPLC connected to a fluorometer. By using this chemical system, .OH yields near the Bragg peak have been measured. Contribution of fragmentations, which are known to be significant near the Bragg peak of high-energy heavy ions, is also discussed by conducting fragmentation simulation.
Heavy ion therapy of cancer has begun because it is much more effective for special cancers that are highly resistant for other typical radiations such as fast electron, 60Co γ-ray, and x-ray. In such therapy, ion is accelerated up to 5 GeV to attain sufficiently long penetration depth into human body (normally 30 cm is necessary) to treat cancer in deep position. Note that there are only a few heavy ion accelerators possessing abovementioned ability in the world, including HIMAC (Heavy Ion Medical Accelerator in Chiba) at NIRS (National Institute of Radiological Science), Japan. While advantages of heavy ion therapy are well-known phenomenologically, details of mechanism in which heavy ion irradiation leads to distinctive biological effectiveness have not been clarified yet. Then, understanding of water radiolysis with heavy ions is necessary because water is main component of human body.
P6
Mechanistic aspects of radiation induced dimerization of thiophene and its disubstituted derivatives
R. Michalski, A. Sikora, J. Adamus, A. Marcinek, Institute of Applied Radiation Chemistry,
Technical University of Łodz, Łodz , Poland
Polythiophenes belong to group of conducting polymers which play an important role in electric and electronic industry. The oxidative polymerization mechanism involves several consecutive steps such as electron transfer, carbon . carbon bond formation and deprotonation. The identification of the reactive intermediates formed upon oxidation of thiophenes is complicated by the high reactivity of their radical cations, the primary products of one-electron oxidation. Therefore, it is useful to investigate the initial steps of the polymerization in low-temperature organic matrices.
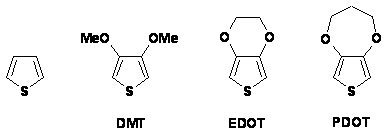
Thiophene, 3,4-dimethoxythiophene (DMT),
3,4-ethylenedioxythiophene (EDOT) and
3,4-propylenedioxythiophene (PDOT) were chosen for this study. The
mixture of ionic liquid 1-butyl-methylimidazolium hexafluorophosphate (BMIMPF6)
and chloroform was used as the low temperature organic matrix. In this type of
matrix annealing of irradiated frozen mixture triggers formation of peroxyl
radicals which can play a role of secondary oxidizing agent. The observed steps
of oxidative dimerization of thiophenes and the role of peroxyl radicals in
.delayed. oxidation of primary radiolysis products of thiophenes are discussed.
P7
Measured rates of fluoride / metal association correlate with rates of superoxide / metal reactions for FeIIIEDTA(H2O). and related complexes
A Mizrahia,b; I. Zilbermanna,b; E. Maimona,b; J. S. Summersc; J. B. Bakerc; C. M. Wilsonc; J. R. Virgac; H. Cohen,b,d D. Meyersteinb,d, aChemistry Dept., Nuclear Research Centre Negev, Beer-Sheva, Israel, bChemistry Dept., Ben-Gurion Univ. of the Negev, Beer-Sheva, Israel, cDepartment of Chemistry and Physics, Western Carolina University, Cullowhee, NC, 28723,USA, dBiological Chem. Dept., The College of Judea and Samaria, Ariel, Israel
The effects of ten paramagnetic metal complexes (FeIIIEDTA(H2O)., FeIIIEDTA(OH)2-, FeIIIPDTA., FeIIIDTPA2-, FeIII2O(TTHA)2-, FeIII(CN)63-, MnIIEDTA(H2O)2-, MnIIPDTA2-, MnII-EDDADP2-, and MnIIPO4.) on F. ion 19F NMR transverse relaxation rates (R2 = 1/T2) were studied in aqueous solutions as a function of temperature. The kinetic parameters (apparent second order rate constants and activation enthalpies) for metal / F. association were determined from the dependence of the observed relaxation enhancements on complex concentration and temperature. Apparent metal / F. association rate constants for these complexes (kapp,F.) spanned five orders of magnitude. In addition, we measured the rates at which O2 . reacts with FeIIIPDTA., MnIIEDTA(H2O)2-, MnIIPDTA2-, and MnII-EDDADP2- by pulse radiolysis. While FeIIIPDTA. is directly reduced by O2 . to the corresponding Fe2+ complex, each of the Mn2+ complexes reacted with formation of an intermediate complex, presumably having a Mn2+.O2 bond. These reactivity patterns are consistent with literature data for similar complexes. With this data both kapp,O2. and kapp,F. available for each of the eight reactive complexes. A plot of log(kapp,O2.) versus log(kapp,F.) for these eight showed a linear correlation with a slope ~1. This correlation suggests that rapid metal / O2 . reactions of these complexes occur via an inner-sphere mechanism where formation of an intermediate coordination complex limits the overall rate.
P8
Quantum beats as a tool to study geminate ion-radical pairs in irradiated solutions
Yu. N. Molin, V. I. Borovkov, P. A. Potashov, V. A. Bagryansky, Institute of Chemical Kinetics and Combustion SB RAS, 3, ul. Institutskaya, Novosibirsk, 630090, Russia
The chemical action of ionizing radiation on nonpolar solutions starts from ionization of solvent molecules that leads to generation of singlet-correlated radical ion pairs. In pulse experiments the geminate recombination of such pairs may result in fluorescence decay modulated due to singlet-triplet evolution (quantum beats) of the pairs prior to recombination. The quantum beats are determined by the same parameters of radical ions (hyperfine couplings, g-values and spin relaxation times) as their EPR spectra. Therefore the quantum beats technique combines the merits of EPR and pulse radiolysis.
The advantages of available installation include (1) high sensitivity due to detection of light emitted, (2) absence of microwave broadening unavoidable for EPR detection of short lived intermediates, (3) better temporal resolution ( 2 ns) as compared with EPR technique and (4) large scale of external magnetic fields (zero to 1 T).
Several examples of application of quantum beat technique will be presented including (1) detection of radical cations of branched alkanes and organo-metallic compounds, (2) investigation of radical cation reactions such as charge transfer and degenerate charge exchange, (3) detection of complexes of amine radical cations and (4) investigation of unusually short relaxation time of c-hexane radical cation.
P9
An ESR study of radiation-chemical transformation of 4,4.,(5.)-di-(tert-butylcyclohexano)-18-crown-6 and its solutions in 1-octanol at 77 K
O. A. Zakurdaeva, S. V. Nesterov, Enikolopov Inst. of Synthetic Polymer Materials of Russian Academia of Science;
V. I. Feldman, Department of Chemistry, Moscow State Universit, Russia
The process of utilization of liquid radioactive waste is simplified by selective removal of fission products, such as 90Sr and 137Cs, which are heat generators and have high specific activity. Crown ether 4,4.,(5.)-di-(tert-butylcyclohexano)-18-crown-6 (DtBuCH18C6) is known to be efficient selective extractant with respect to 90Sr cations. 1-Octanol was frequently used as a solvent for the crown ether demonstrating high performance with respect to strontium recovery. From this point of view, the radiation resistance of extractant is of great importance, in particular, in terms of stability of the macrocyclic structure of crown ether. It should be noted that macrocycle cleavage results in drastic fall in extraction ability of crown ether. The transformations occurring in DtBuCH18C6 solution in octanol and radiation resistance of DtBuCH18C6 were virtually not investigated.
The aim of the present study is to identify the structure of paramagnetic intermediates stabilized in pure DtBuCH18C6 and its solutions in 1-octanol irradiated at 77 K by X-rays and to estimate the probability of macrocyle cleavage. ESR spectroscopy was used as a method of investigation. DtBuCH18C6, 1-octanol, 0.1 M and 1.0 M DtBuCH18C6 solution in 1-octanol were chosen as objects of investigation.
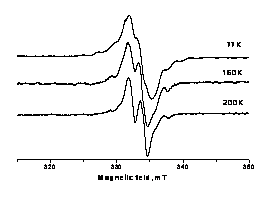
|
ESR spectra of 1.0M DtBuCH18C6 solution in 1-octanol irradiated with X-ray at 77 K |
ESR spectra of DtBuCH18C6 and its solutions in octanol were recorded in the temperature range of 77 - 240 K. Irreversible transformation of ESR spectra of DtBuCH18C6 irradiated at 77 K was found above 200 K. Analysis of spectra before and after the transformation demonstrated the presence of, at least, two paramagnetic species: macrocyclic radical .CH2-ĊH-O- and radical -ĊH-C(=O)H. These radicals were also observed in irradiated crown-containing octanol solutions, in addition to RCH2ĊH-OH resulting from the solvent. The radical products of polyether ring cleavage were observed both in pure DtBuCH18C6 and its solutions.
The work was supported by the Russian Foundation for Basic Research, project № 09-03-00877a.
|
P10
Study of primary charge transfer reactions of radical ions in irradiated nonpolar solutions by MARY spectroscopy
V. N. Verkhovlyuk, N. V. Arabadzhi, A. B. Doktorov, D. V. Stass, Yu. N. Molin, Institute of Chemical Kinetics & Combustion, Novosibirsk
V. A. Morozov, International Tomography Center, Novosibirsk, Russian Federation, Russia
Study of properties of radical ion intermediates produced in liquid solution under irradiation is a very important aspect of radiation and spin chemistry. The main primary elementary process after irradiation of solution is the irreversible charge transfer reaction between the radical cation of the solvent and a neutral molecule of hole acceptor. This process is very important for constructing radical ion pairs which can be detected by some spin chemistry methods. Quite effective instruments for this purpose are methods based on studying of magnetosensitive fluorescence from recombining radical ions. MARY (Magnetic Affected Reaction Yield) spectroscopy is one of such methods which allows us to study very short-lived radical ions with lifetimes down to several nanoseconds and their reactions. But, for correct interpretation of experimental data one has to use appropriate theoretical description of spin evolution of radical pairs with taking into account charge transfer reaction. This work is devoted to development of the theoretical approach for describing MARY experimental data with taking into account the charge transfer reaction, and applying this approach to simulation of some typical experimental systems in radiation chemistry. We shall discuss the processes of the chemical decay of radical ions and the process of charge transfer with instantaneous change in the magnetic structure of the radical ions.
P11
Unusual photochemistry of the methyloxirane radical cations in freonic matrices
I. D. Sorokin et al., Moscow State University, Russia
Mechanisms of reactions under the influence of light on the methyloxirane (MO) radical cations (RC) obtained by x-ray irradiation and stabilized in freonic matrices at 77 K are investigated by the ESR and UV-VIS spectroscopy as well as quantum chemistry (TD DFT, PBE1).
The positions of two peaks in the UV-visible spectra are similar in both freons (λ1 ≈ 435 nm for «band 1» and ≈ 530 nm for «band 2»). The absorption coefficients are estimated to be 1.9 l/(mol cm) and 3.3 l/(mol cm) respectively, while the quantum yields of the reactions taking place under the influence of light (λ1 = 436 nm and λ2 = 546 nm) are approximately φ1 = 0.03 and φ2 = 0.02.
As direct measurement of the hyperfine couplings in the ESR spectra proved problematic due to the complicated spectra, modeling of the spectra was carried out. The modeling was based on the results of the TD DFT calculations with further optimization for the better accommodation of experimental results, the most intriguing of which is the rather large doublet coupling constant arising in the ESR spectra corresponding to the 435 nm peak in the UV-VIS spectra. It.s also necessary to mention that the quantum chemical calculations predict potential minima for two «ring-closed» forms for the MO RC (differentiated by the C-C bond length: 1.46 . versus 1.72 . which is the one with the large doublet coupling) as well as two «ring-open» forms.
With the modeling being successful in separating individual experimental spectra attributed to three different forms of the MO RC it is discovered that the conclusions of Shida et al. [1] are erroneous and the interpretation of the reversible photoinduced changes as cis-trans isomerisation of the «ring-open» forms of the RC must be substituted with an explanation taking a process that involves the «ring-closed» forms into account.
ESR spectrum with the large doublet due to the «ring-closed» form with a longer C-C bond which arise from photochemical excitation (as opposed to the other cyclical form that doesn't exist at T = 77 K) while the original spectrum observed immediately after irradiation is attributed to the mixture of the «ring-open» forms of the RC.
The work was supported by the grant of RFBR (07-03-00105) and the program of Presidium of RAS ChD-01.
Reference:
1 K. Ushida, T. Shida, K. Shimokoshi: J. Phys. Chem. 93, 5388 (1989)
P12
Imaging of multi-ionization spurs from low LET radiations absorbed by polymers
Z. P. Zagorski, W. Gluszewski, Department of Radiation Chemistry and Technology,
Institute of Nuclear Chemistry and Technology, 03-195 Warsaw, Poland
Distribution and kind of spurs resulting from absorption of low LET radiation was discussed practically only for the case of water. The early developments have clearly distinguished the presence of single ionization spurs, as the origin of radical products of water radiolysis and multi-ionization spurs yielding molecular products, i.e. H2 and H2O2. Mechanism of spurs formation was shown by Henglein in his .Strahlenchemie. monograph. Limitation of that picture to aqueous systems is not justified, because it is valid for any low Z medium and we expect the same phenomena in polymers as well. 80% of deposited energy is distributed in single spurs and the rest in multi-ionization spurs, formed as congestion of several ionizations very close to one another. Almost reversed situation is in the case of high LET radiations, where the most of energy is forming multi-ionization spurs, as in the case of alpha radiation. The latter produces in water mainly H2 and H2O, due to the domination of overlapping of ionization spurs and low yield of radical products. The same phenomenon of concentrated deposition of energy in the zone of multi-ionizations must occur in any condensed phase. Indeed, polycarbonates are applied to the determination of radon. Alpha particle absorbed on the surface of polycarbonate film causes local damage prone to etching by NaOH solution. Resulting micro-holes are easily counted. Single ionization spurs are not affected by etching. In our case of low LET, the participation of multi-ionization spurs is lower, but they are present and we have applied the similar technique, taking into account the facts of smaller size of multi-ionization spheres and the fact of reaching the etching solution only to spurs present at the surface of the film. Pictures in the text of the presentation show the effects of experiments. Semiquantitative estimate of yields confirms the expected, ~20% yield of multi-ionization spurs. The dominating effects by low LET radiations (EB and γ) consists in chemical effects starting in single ionization spurs (crosslinking and degradation), present results show, it is worthwhile to look into effects caused by multi-ionization spurs, e.g. sites of higher degradation, of specific products formation, and/or formation of gases.
P13
Radical cations and neutral radicals produced by irradiation of crown ethers in Freon matrices
O. A. Zakurdaeva, Enikolopov Institute of Synthetic Polymer Materials of RAS
S. V. Nesterov, Enikolopov Institute of Synthetic Polymer Materials of RAS
V. I. Feldman, Department of Chemistry, Moscow State University, Russia
Crown ethers are well known as selective extractants useful for liquid radioactive waste reprocessing. The understanding the mechanism of crown ether radiolysis is of great importance for modeling and prediction of their behavior under ionizing radiation action. A lot of experimental data on the radiolysis of organic oxygen-containing cyclic compounds demonstrates that the reactions of primary radical cations play important role in the radiation-induced processes. These data were obtained by Freon matrix technique, which gives the opportunity for stabilization of radical cations and investigation of their post-irradiation transformations.
The aim of this work was to identify paramagnetic species in radiolysis of unsubstituted crown ethers at 77 K and to study the mechanism of their transformations. 12-Crown-4, 15-crown-5 and their solutions in Freons (CFCl3, CFCl2CF2Cl, CF3CCl3, с12C4 and с15C5 = 0.02÷1.3 mol %) were irradiated by X-rays and the radicals were characterized by ESR. The post-irradiation reactions were also investigated.
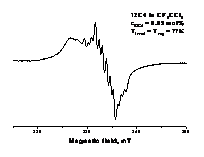 We have obtained first experimental evidence for the
stabilization of primary macrocyclic radical cations (see figure) upon
irradiation of 12-crown-4 in the CF3CCl3 matrix (с12C4
= 0.05 mol %), whereas only neutral macrocyclic radicals -СН2-.СН-О-
were stabilized in CFCl3 and CFCl2CF2Cl
matrices in the studied range of crown ether concentrations. It was proposed
that formation of neutral radicals occurred as a result of deprotonation of
primary radical cations and most likely caused by the presence of neutral
dimmers even at low crown ether concentration. The irradiation of pure
12-crown-4 led to the stabilization of two types of neutral radicals:
macrocyclic radicals -СН2-.СН-О-
and acyclic -.СН-СН=О radicals.
We have obtained first experimental evidence for the
stabilization of primary macrocyclic radical cations (see figure) upon
irradiation of 12-crown-4 in the CF3CCl3 matrix (с12C4
= 0.05 mol %), whereas only neutral macrocyclic radicals -СН2-.СН-О-
were stabilized in CFCl3 and CFCl2CF2Cl
matrices in the studied range of crown ether concentrations. It was proposed
that formation of neutral radicals occurred as a result of deprotonation of
primary radical cations and most likely caused by the presence of neutral
dimmers even at low crown ether concentration. The irradiation of pure
12-crown-4 led to the stabilization of two types of neutral radicals:
macrocyclic radicals -СН2-.СН-О-
and acyclic -.СН-СН=О radicals.
The data suggest that the radical cations were principal precursors of neutral radicals observed for 12-crown-4 and 15-crown-5 in Freons as well as in neat crown ethers irradiated at 77 K.
The work was supported by the Russian Foundation for Basic Research, project number 09-03-00877a.
P14
The radical scavenging properties of tetrathiotungstate
I. Popivker,a,b, I. Zilbermanna,b, E. Maimona,b, H. Cohenb,c, D. Meyersteinb,c, a Chemistry Dept., Nuclear Research Centre Negev, Beer-Sheva, Israel, bChemistry Dept., Ben-Gurion Univ. of the Negev, Beer-Sheva, Israel, c Biological Chem. Dept., Ariel University Center of Samaria, Ariel, Israel
Tetrathiotungstate, WS42-, is used as its close analogue, MoS42-, as a therapeutic agent for the Wilson disease(copper excess) due to its capacity to form very stable Cu(II) complexes. The former was lately shown to be even more efficient than the latter as it hydrolyzes two orders of magnitude slower1.
Furthermore WS42- was shown to have (as its molybdenum analogue does) potent antiangiogenic effects in multiple animal models and in human cancer clinical trials2.
In an earlier study we have shown the radical scavenging properties of MoS42- 3.
In this study, using the pulse radiolysis technique, the reactions of WS42- with several oxidizing and reducing species were investigated.
O2· . and CO2· . did not react with the tungsten complex (contrary to what was observed for its molybdenum analogue), while the oxidizing species ·OH, CO3·. and ·CH2(CH3)2COH react with WS42- in fast reactions, k(·OH) = 1 x 1010 M-1s-1; k (CO3·.) = 2.7 x 108 M-1s-1; k (·CH2(CH3)2COH) = 1.8 x 108 M-1s-1.
The aquated electron, a strong reducing reagent (-2.0 V vs. NHE) reduces the tungsten complex in a fast reaction with k(e-aq) = 1.2 x 1010 M-1s-1.
The results obtained fit the fact that while thiols are relatively easy to oxidize, W(VI) is reluctant to one electron reduction compared to Mo(VI).
Detailed kinetic data will be presented.
References:
1. V. E. Lee, J. M. Schulman, E. I. Stiefel, C. Coyle-Lee: J. Inorg. Biochem. 101, 1707 (2007)
2. G. Hou, R. Dick, C. Zeng, G. J. Brewer: Trans. Res. 149, 260 (2007)
3. I. Popivker, et al: PULS.2008, Krakow (2008)
P15
Yield of .OH in N2O saturated aqueous solution
E. Janata, Helmholtz-Zentrum Berlin für Materialien und Energie GmbH, Solar Energy Research
Glienicker Straße 100, 14109 Berlin, Germany
Nitrous oxide is in widely use for converting reducing hydrated electrons into oxidizing hydroxyl radicals. The yield of hydroxyl radicals resulting from the very fast reaction of hydrated electrons with nitrous oxide in aqueous solution has been re.determined using the method of pulse radiolysis. For solutions saturated with nitrous oxide the half-life time of this reaction is about 3 ns, i.e., this reaction occurs on a time scale where spur reactions are still underway. At these times, the yield of hydrated electrons is definitely larger than the known value of 0.269 µmol J.1 (2.6 molecules/100 eV) at about 100 ns after irradiation. Mimicking the experimental absorption vs. time curves of the hydrated electron at 700 nm for solutions either being purged with argon or being saturated with nitrous oxide reveals that hydroxyl radicals, due to the reaction of hydrated electrons with nitrous oxide, are generated with a yield of 0.33 µmol J.1 (3.2 molecules/100 eV). Compared with the normally used value of 0.269 µmol J.1 (2.6 molecules/100 eV), this new value is 23 % larger.
P16
Large inverse isotope effects in the hydrogen atom abstraction from L-Rh(III)-H/D macrocyclic complexes by methyl radical in aqueous solution
L. Kats, Chemistry Dept., Ben-Gurion Univ. of the Negev, Beer-Sheva, Israel
E. Mimon, Chemistry Dept., Nuclear Research Center Negev, Beer-Sheva, Israel
D. Meyerstein, Chemistry Dept., Ben-Gurion Univ. of the Negev, Beer-Sheva and Biological Chemistry Dept., Ariel University Center of Samaria, Ariel, Israel
The reactions ![]() where
M is transition metal cation and x = halides, H, alkyls etc.) are of importance
in a large variety of radical induced catalytic processes. The factors
affecting the kinetics of these reactions have still not been fully elucidated.
It was decided to measure the kinetics of the reactions:
where
M is transition metal cation and x = halides, H, alkyls etc.) are of importance
in a large variety of radical induced catalytic processes. The factors
affecting the kinetics of these reactions have still not been fully elucidated.
It was decided to measure the kinetics of the reactions:![]()
(where L = 1,4,8,11-tetraazacyclotetradecane and R. an alkyl radical) as the hydride ligand induces no steric hindrance. The first radical studied was the methyl radical, formed radiolytically in aqueous solutions. The rate constants of the latter reactions are 1.3*105 M-1s-1 and 5.6*104 M-1s-1 for the trans- and cis- isomers of the complex respectively. These low rate constants might be due to the RhIII-H bond strength. In order to verify this assumption it was decided to measure the isotope effects of these reactions. Surprisingly, large inverse isotope effects are observed:
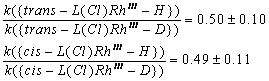
These isotope effects indicate that the radical first forms a metal-carbon bond followed by reductive elimination of methane.
P17
Effects of silica particles on reactions of radiolytic products of water
Y. Kumagai,1,2 R. Nagaishi,1 R. Yamada,1Y. Muroya,2 Y Katsumura1,2
1Japan Atomic Energy Agency, 2The University of Tokyo, Japan
Increase in the yields of H2 production and reduction of oxidative metal ions has been reported in mixed systems of silica particles and aqueous solutions.1,2 The understanding of the mechanisms of this effect enables to control the radiation-induced reactions. Concerning this effect, radiolysis of water in the presence of silica has been studied.3 Little is known of the reaction leading to increase in production of H2 or reduction of metal ions.
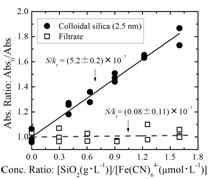 Thus the effect of silica on radiation-induced reactions was
studied by pulse-radiolysis using pulsed electron beam from LINAC at UTNL (10 ns,
10 Gy/pulse). Decay kinetics of hydrated electron and formation kinetics of
Fe(CN)63- produced by reaction of OH and Fe(CN)64-
were studied in the presence of nano-sized colloidal silica (2.5 ±0.5 nm in
diameter) prepared by hydrolysis of Si(OC2H5)4.
Thus the effect of silica on radiation-induced reactions was
studied by pulse-radiolysis using pulsed electron beam from LINAC at UTNL (10 ns,
10 Gy/pulse). Decay kinetics of hydrated electron and formation kinetics of
Fe(CN)63- produced by reaction of OH and Fe(CN)64-
were studied in the presence of nano-sized colloidal silica (2.5 ±0.5 nm in
diameter) prepared by hydrolysis of Si(OC2H5)4.
In Ar saturated water added 50 mM t-butanol as scavenger of OH radical, little change in decay kinetics of hydrated electron was observed. In the aqueous solution of K4Fe(CN)6 saturated by N2O to convert hydrated electron to OH radical, decrease in absorbance of Fe(CN)63- was observed as the concentration of colloidal silica increased (Figure 1). This decrease is considered to be due to the interaction of silica particles with OH radical and it competes with reaction of Fe(CN)64- to reduce the yield of Fe(CN)63-.
References:
 1. J. A. LaVerne, S. E. Tonnies: J. Phys. Chem. B., 107, 7277 (2003)
1. J. A. LaVerne, S. E. Tonnies: J. Phys. Chem. B., 107, 7277 (2003)
2. R. Nagaishi, Z. Yoshida, R. Yamada, Y. Hatano: Radiat. Phys. Chem., 75, 1051 (2006)
3. N. M. Dimitrijevic, A. Henglein, D. Meisel: J. Phys. Chem. B., 103, 7073 (1999)
P18
Radiolysis simulation of water confined in porous silica
H. Ouerdane, B. Gervais, CIMaP, UMR CEA-CNRS-ENSICAEN-UCBN 6252, Boulevard Henri Becquerel, BP 5133, F-14070 Caen, France
M. Beuve, A. Colliaux, H. Zhou, LIRIS, IPNL, UMR IN2P3-CNRS-UCBL 5822, Villeurbanne, F- 69622, France
J.-P. Renault, CEA/Saclay, DSM/DRECAM/SCM/URA 331 CNRS, 91191 Gif-sur-Yvette Cedex, France
The radiolysis of water is a phenomenon occurring in a rich variety of contexts such as cooling of nuclear plants, nuclear waste storage and irradiation of living cells. A lot of work in this field has been performed. Yet many questions remain unanswered, in particular when radiolysis occurs near interfaces. The study of the radiolysis of water confined in submicron volumes within a porous matrix is representative of this class of problems. Our approach aims at a microscopic simulation of radiolysis in liquid water confined in a surrounding porous silica matrix. Our model accounts for both the geometry of the problem and the projectile (photon, electron or ion) characteristics. The method is based on Monte Carlo simulation of all events generated by radiation in a simulation box containing several pores surrounded by silica. We consider the problem of an array of liquid water cylinder confined in silica. The simulation is divided in three stages: physical, physico-chemical and chemical stages; each of which corresponds to processes of different nature occuring typically on different time scales. The physical stage describes the interaction of the projectile with the medium in terms of electronic excitation and ionisation. In the case of ionising events, it describes the resulting electronic cascade, which ionizes either the water molecules or the silica. We take into account the electronic structure of both materials. We show that the transport near the interface is controlled by the relative electron affinity of both media which determines the flux of electron toward water or silica. On the other hand, the net balance of electron flux depends on the escape length, which is itself controlled by the electron-phonon coupling in each material. The physico-chemical stage accounts for intra-molecular reorganization and quick rearrangements with neighboring molecules. This stage is dominated by proton transfer in liquid water. Finally the chemical stage, simulated by means of the kinetic Monte Carlo method, accounts for the diffusion of molecular products and radicals generated during the preceding stages, over a time scale from a few picoseconds to milliseconds.
P19
Organic free radicals in superheated water probed by muon spin spectroscopy
P. W. Percival, J.-C. Brodovitch, Simon Fraser University, Burnaby, British Columbia, Canada
B. M. McCollum, Mount Royal College, Calgary , Alberta, Canada
K. Ghandi, Mount Allison University, Sackville, New Brunswick, Canada
The study of organic chemistry in superheated water is motivated by many different applications: geochemical production of fossil fuels, biology of submarine volcanic vents, corrosion in pressurized water nuclear reactors, destruction of hazardous materials, and .green. industrial processes. However, fundamental investigation of chemical reactions requires the detection and monitoring of intermediates, often transient free radicals, which are difficult to detect under the extremes of pressure and temperature that define near critical and supercritical aqueous systems. We have demonstrated the ability to study H atom and free radical chemistry in aqueous systems up to 400.C and 400 bar [1], using the exotic atom muonium (Mu) as an effective light isotope of hydrogen. Unlike the optical detection methods usually employed in pulse radiolysis studies, muon spin spectroscopy is sensitive only to the transient under study (Mu or a free radical incorporating it), and is unaffected by environmental effects (scattering of light, change in extinction coefficient, etc.). Moreover, by measuring muon and other nuclear hyperfine constants it is possible to characterize free radicals in a manner similar to electron spin resonance spectroscopy, which so far has not been applied to studies under extreme hydrothermal conditions. Our work includes studies of dehydration of alcohols and the enolization of acetone. In both cases the production of muoniated radicals by Mu addition to an unsaturated compound serves to .trap. that species for in situ identification. Other work focuses on the radical itself. For example, we have determined muon and proton hyperfine constants for the tert-butyl radical in water over a wide range of temperatures. By analyzing the temperature dependence it is possible to deduce details of intramolecular motion as well as possible interactions with the solvent.
Reference:
[1] P. W. Percival et al.: J. Am. Chem. Soc. 127, 13714 (2005)
P20
Radiation-induced reactions of carboxymethyl cellulose in an aqueous solution
S. Saiki, N. Nagasawa, A. Hiroki, N. Morishita, M. Tamada, Quantum Beam Science Directorate,
Japan Atomic Energy Agency
H. Kudo, Y. Katsumura, Graduate School of Engineering, The University of Tokyo, Japan
Carboxymethyl cellulose (CMC) at highly concentrated aqueous solution undergoes crosslinking reactions by ionizing irradiation, though polysaccharides and their derivatives are generally radiation-degradation type polymers. Through these crosslinking reactions, CMC hydrogel, which is able to absorb one hundred times water as dry weight, can be formed. Radiation-induced reactions of polymer aqueous solution, such as crosslinking, degradation and so on, originate mainly from water radiolysis products, especially OH. In this topic, focusing on CMC radicals produced by reactions with OH, radical behavior was observed by ESR method to understand radiation-induced reaction mechanism of CMC aqueous solution. At first, to identify CMC radical sites produced by reactions with OH, direct observation of CMC radicals in an aqueous solution was tried by ESR method using photolysis of hydrogen peroxide. As a result, the spectra of these CMC radicals were succeeded to observe, and were identified as radicals located on carbon atoms of carboxymethyl groups. Next, CMC aqueous solution saturated N2O after electron beam irradiation was measured by ESR method. Then, long-lived radicals were observed, and the spectra were coincident with the spectra observed using photolysis of hydrogen peroxide. This means that long-lived radicals are radicals located on carboxymethyl groups. Furthermore, decay behavior of ESR spectra of long-lived CMC radical was observed. In this presentation, the dependency on CMC concentration of the decay behavior will be discussed.
P21
The density effect on solvated electron in sub- and supercritical water and methanol
Y. Yan, School of Engineering, The University of Tokyo
Y. Katsumura, School of Engineering, The University of Tokyo
Advanced Science Research Center, Japan Atomic Energy Agency
M. Lin, Advanced Science Research Center, Japan Atomic Energy Agency
Y. Muroya, School of Engineering, The University of Toky, Japan
The optical absorption spectra of solvated electron in sub- and supercritical water and methanol are measured by electron pulse radiolysis techniques as a function of water (heavy) and methanol density. In agreement with previous work, the position of the solvated electron absorption maximum (EAmax) is found to shift only slightly to lower energy with decreasing density over the critical point temperature (tc). In both water and methanol, the temperature dependence of EAmax in sub- and supercritical fluids (SCF) reveals that, at a fixed pressure, EAmax decreases monotonically with increasing temperature in passing through the liquid-SCF phase transition at tc, but exhibits a minimum at a fixed density as the fluids passes above tc into SCF. These behaviors can be understood by means of simple microscopic arguments based on the changes that occur in the water properties and water structure in the sub- and supercritical water regimes. Most importantly, the role of local density and molecular configurational fluctuations (associated with criticality) in providing pre-existing polymeric clusters which act as trapping sites for the excess electron is a pivotal point in the interpretation of the data.
P22
Direct damage to the backbone of DNA oligomers is influenced by the OH function at strand ends, by the type of base, and by the base context
K. K. K. Sharma, W. A. Bernhard, Dept. of Biochemistry & Biophysics, University of Rochester, NY, 14642 USA
Radiation damage to the deoxyribose of DNA results in stable end products consisting of strand breaks (sb) and free base release (fbr). Understanding the reaction mechanisms that lead to these products, following the direct action of ionizing radiation with DNA, is central to determining risk due to radiation exposure at low dose. The mechanism has been presumed to be straightforward: ejection of an electron from the DNA backbone produces a radical cation that deprotonates to yield a neutral carbon centered deoxyribose radical and, in the presence of water, these neutral radicals react to give sb and fbr. One would expect, therefore, that the sb/fbr site should be independent of the base at that site. By studying oligodeoxynucleotides of known sequence, we have found that this is not the case.
Transparent films were prepared from palindromic deoxyoligonucleotides of d(CTCTCGAGAG), d(CTCTCGAGAGp), d(CTCTCTTAATAATTATAATTATTAAGAGAG). d(pCTCTCGAGAGp), d(GAGAGCTCTC), d(ACGCGCGCGT), d(AACGCGCGCGTT), d(CTCTCTTAATATTAAGAGAG), and the DNA in these films was hydrated to ~2.5 waters per nucleotide. The films were irradiated at RT under air using X-rays generated by a tungsten tube operated at 70 kV and 20 mA. The X-irradiated oligodeoxynucleotide films were immediately dissolved in nuclease free water and stored at 277 K for 24 hrs. Unaltered free base release was measured using HPLC. Yields of free base release were based on a target mass consisting of the DNA and one counterion + 2.5 H2O/nucleotide. The yields of each base, G(C), G(G), G(T), and G(A) were determined for each sequence.
The observed changes in yields lead to the following conclusions: (i) phosphorylating the OH function quenches the end effect, (ii) the base at one end of the oligomer influences base release at the other end but only in shorter (10 bp) oligomers, (iii) the end effect is influenced by the base at the end and by the bases proximal to it, and (iv) the release of bases from internal positions is influenced by the base and its base context.
Supported by PHS Grant 2-R01-CA32546 of the NCI.
P23
A new technique for studying radiation induced strand breaks in DNA: tunable filtration of DNA oligomers through nanoporous silicon membranes
P. J. Black, University of Rochester, Rochester, 14620, USA
We have taken advantage of the unique properties of Simpore membranes to achieve favorable filtration cutoffs on microgram quantities of DNA. Simpore membranes (Simpore, Inc., Rochester, NY) are a unique nanoporous silicon material with well defined pore size distributions and selectable maximum diameters ranging from 10 nm to 50 nm. In addition to small pore diameters, these membranes are extraordinarily thin (5 to 20 nm). Because the ratio of pore diameter to pore length is > 1, these membranes have exceptional filtration properties.
The experimental setup consisted of a membrane sandwiched between two drops of phosphate buffer solution (PBS) containing variable concentrations of NaCl. The top drop (retentate) contained DNA ladder, e.g., 10 to 300 base pairs (bp), in a solution volume of 20 microliters. DNA would then freely diffuse into the bottom drop (filtrate). This was monitored using gel electrophoresis. In our preliminary experiments, we found that membrane permeability was a function of DNA size and that the permeability was tunable through adjustment of the salt concentration of the DNA solution. Salt concentration influences the membrane surface charge, which is strongly negative in the absence of salt. In addition, as the salt concentration is lowered, the negative charge of DNA is increasingly exposed. Thus, at low cation concentrations, the negatively charged membrane surface repels DNA. At low NaCl levels, we observed a filtration cutoff at ~20 bp. At high salt levels no filtration cutoff up to 100 bp of duplex DNA was observed.
Our findings suggest that Simpore membranes have the potential of opening doors to an exciting set of experiments that will shed new light on the chemistry and physics of strand break formation in DNA due to the direct effect of ionizing radiation.
Supported by PHS Grant 2-R01-CA32546 of the NCI.
P24
Amino acid peroxyl radicals: formation and reaction with ascorbate
A. S. Domazou, V. D. Zelenay, W. H.Koppenol, Institute of Inorganic Chemistry, Swiss Federal Institute of Technology, CH-8093 Zürich, Switzerland,
J. M. Gebicki, Free Radical Biochemistry Group, Department of Biological Sciences, Macquarie University,
Sydney, NSW 2109, Australia
Proteins are significant targets for partly reduced oxygen species in vivo. This results in random formation of radicals on the amino acid residues (AA·) of the protein, which in turn, in the presence of oxygen, can yield the corresponding peroxyl radicals (AAOO·). Both radical types can cause further biological damage.
We studied the N-acetylamide derivatives of the amino acids glycine, alanine and proline as models of these residues in proteins. We generated the amino acid radicals specifically by reaction with hydroxyl radicals produced in solutions irradiated with 2 MeV electrons in the presence of N2O. In the absence of oxygen the amino acid radicals decayed with rate constants in the narrow range (0.9-1.3)´109 M-1s-1, while in the presence of oxygen they were converted very rapidly to the corresponding peroxyl radicals with rate constants that vary between 6.3´108 and 5.5´109 M-1s-1, depending on the amino acid. The corresponding N-acetylated amino acids were also studied and showed similar behaviour but with slightly smaller rate constants.
Antioxidants are able to repair tyrosyl and tryptophanyl radicals in various proteins in vitro.1,2 For ascorbate, the principal endogenous biological antioxidant, we have measured rate constants in the range 105-108 M-1s-1.2
The peroxyl radicals of all amino acids studied here were reduced by oxidizing ascorbate to the ascorbyl radical. The reaction was followed at 360 nm, where ascorbyl radical has an absorption coefficient of 3300 M-1cm-1, and the derived rate constants were all close to 107 M-1s-1. However, the spontaneous decay of peroxyl radicals is also fast and competes with the reaction with ascorbate. It is to be stressed that reaction of AAOO· and ascorbate gives rise to hydroperoxides (AAOOH) that are also reactive molecules. Our study suggests that reaction with protein radicals may be responsible for the ascorbate loss reported in organisms exposed to oxidative stress.
References:
1.B. M. Hoey, J. Butler: Biochim. Biophys. Acta 791, 212, (1984)
2.A. S. Domazou, W. H. Koppenol, J. M. Gebicki: Free Radical Biol. Med. 46, 1049, (2009)
P25
Radiolytically generated hydroperoxides in aqueous micellar solutions
J. L. Gebicki, P. Meisner, Institute of Applied Radiation Chemistry, Faculty of Chemistry,
Technical University of Łodz, Łodz, Poland
Hydroperoxides, molecular products of the oxidation of e.g. lipids and proteins, can further decompose into secondary oxidation products, which are reactive towards other compounds present in food or biological samples. Thus hydroperoxides are included into the class of so called reactive oxygen species which may contribute to oxidative stress. Hydroperoxides may also form on surfactant molecules, and hence may interfere either with numerous determinations run in the systems containing surfactants or with the mechanism of the action of antioxidants delivered in diet supplements which usually contain surfactants as additives.
The ferrous oxidation-xylenol orange method (FOX method) appears to be one of the most convenient methods for determination of hydroperoxides due to its simplicity, sensitivity and reasonable reproducibility. Our studies have shown that this method can be effectively used to determine hydroperoxides in the presence of different surfactants either below or above critical micelle concentration.
We have applied the FOX method to determine hydroperoxides formed on surfactant molecules upon exposure to the daylight or gamma irradiation. One of the conclusions derived from these studies is that surfactants as well as their solutions should be kept in dark to avoid the accumulation of peroxides.
We have also used the FOX method to determine hydroperoxides produced radiolytically in aqueous solutions containing amino acid and flavonoid or protein and flavonoid. This was aimed to conclude whether or not the flavonoids are able to break the peroxidation chain.
P26
Physicochemical properties of berberine and palmatine upon binding to DNA
M. Marszalek, M. Wolszczak, Institute of Applied Radiation Chemistry, Technical University of Łodz, Wroblewskiego 15, 93-590 Łodz, Poland
Berberine and palmatine are two important members of quaternary protoberberine alkaloids.

Their salts exist in many Chinese herbal medicines, such as Rhizoma coptidis and Caulis mahoniae1. The ability of some protoberberine alkaloids to act as topoisomerase II poisons is linked to their anticancer activity. These alkaloids possessing also antimicrobial, antidiarrheal and cardiovascular activities, are useful for the development of more efficient DNA-binding agents. The aim of our study was to investigate the influence of binding to DNA on physicochemical properties of these drugs. Binding properties have been studied using absorption, emission, DNA melting, viscometric and fluorescence polarization experiments. The results indicate partial intercalation of both alkaloids into DNA helix, leading to enhanced fluorescence quantum yield and longer lifetimes of the excited state. No berberine phosphorescence or T-T absorption were observed in the aqueous solution. Once bound to DNA, photoexcitation of berberine at 351 nm by laser pulse, leads to the triplet state with high efficiency. The triplet is detected by its transient absorption, with maxima at 425 nm and 560 nm. Its deactivation occurs in the millisecond timescale.
Using pulse radiolysis we recorded absorption spectra of reduced and oxidized forms of alkaloids in aqueous solutions. The same intermediates as in the case of aqueous solutions are formed for the reduction by hydrated electron of drugs intercalated into DNA. We have found that the reaction of hydrated electrons with the scavenger molecules residing in the potential field of negatively charged DNA is strongly inhibited. Preliminary results on degradation DNA by ionizing radiation in the presence of berberine and palmatine will also be discussed.
Reference:
1.Q. Liu, Y. Liu, Y. Li, S. Yao: J. Sep. Sci. 29, 1268 (2006)
P27
Influence of peptide/protein binding on the formation of direct-type damage in DNA
A. R. Peoples, W. A. Bernhard, Department of Biochemistry and Biophysics, University of Rochester, NY, USA
DNA-binding polycations, most notably histones, are known to confer protection to DNA against radiation induced damage. Until recently, DNA protection was believed to be entirely against the indirect effect. The purpose of this study is to test if strong binding between polycations and DNA confers significant radioprotection against the direct effect and to understand how polycations modify the direct-type DNA damage. Our approach is to quantify direct-type damage in terms of free radical trapping and unaltered free base release by the DNA. This approach takes advantage of the fact that deoxyribose free radicals are unstable intermediates in the reaction pathways that lead to strand breaks and free base release.
Solid state films were prepared from the dodecamer d(CGCGAATTCGCG)2 alone, peptide (KKKKY) alone, and dodecamer‑peptide complex. The films were hydrated under a relative humidity of 8%, which was assumed to give Γ @ 2.5 mole water/mole nucleotide for the dodecamer alone. Free radical yields were measured by EPR at 4 K for films with DNA-phosphate:lysine ratios of 1:0, 2:1, 1:1, 1:2 and 0:1. We found free radical yields, in nmol/J, of 346 ± 19, 598 ± 8, 793 ± 25, 900 ± 43 and 825 ± 31, respectively. The yields were calculated based on a presumed target mass consisting of the dodecamer, 2.5 waters per DNA-phosphate, Lys binding to up to half of the DNA-phosphates with Cl- associated with the unbound Lys, Na+ bound to the remaining DNA-phosphates, and 5.5 waters per Lys. These results suggest that free radical trapping in the dodecamer is enhanced by peptide binding.
Experiments are in progress using HPLC to measure base release. The effects of proteins such as histones binding to DNA, are being studied by EPR and HPLC. A comparison of base release with free radical trapping is expected to provide new insights into the mechanisms by which polycation/protein binding influences direct-type DNA damage.
Supported by PHS Grant 2-R01-CA32546 of the NCI.
P28
Radiolytical oxidation of ascorbic acid in aqueous solutions
M. Szymanska-Owczarek, J. L. Gebicki, Institute of Applied Radiation Chemistry, Faculty of Chemistry,
Technical University of Łodz, Łodz, Poland
Ascorbic acid, AsA (vitamin c), has been widely studied as an antioxidant or as an initiator of some technological processes, for example polymerization or nanoparticles formation. AsA can be easily oxidized to ascorbyl radical, in the first stage, and to dehydroascorbic acid, DHA, in the second stage. It has been found that several different ascorbyl radicals are formed during AsA oxidation but the main radical exists as the anion with the unpaired electron delocalized on a highly conjugated tricarbonyl system.
Absorption spectrum of ascorbyl radical shows two bands with maxima at 300 and 360 nm, however only that at 360 nm is proportional to the dose and thus this wavelength was chosen for observations. We studied the oxidation of AsA by the following oxidizing radicals generated by the pulse radiolysis method ·OH, (SCN)2-·, Cl2-·, N3· and NO2·. The observed dependence of the yield and the formation rate of the AsA radical on the reduction potential of the oxidizing radical is discussed.
The results obtained in water are compared with those obtained with AsA enclosed in the water pools of reverse micelles formed by AOT in n-heptane or by Igepal CO-520 in c-hexane. Somewhat surprising observation of different ascorbyl radical in pulse irradiated reverse micelles containing DHA is also commented.
P29
Study on microbial contaminants from biodeteriorated books, archives and manuscripts
M. Constantin, M. Alexandru, L. Trandafir, V. Moise, IRASM Irradiation Technology Center, .Horia Hulubei. National Institute for Physics and Nuclear Engineering, 407 Atomistilor Str.,
077125, Magurele, Ilfov County , Romania
The interest on the application of ionizing radiation treatment to books or papery, dates from 1960, when the radiation resistance of a high number of bacteria and mould strains was tested in the context of the conservation of the cultural heritage.
The field of application of irradiation technology for books and papery disinfection has witnessed a long time pause caused by the reticence for this method and because an alternative methods for sterilizations treatment, fumigant gases (EtO).
Using different microbiological methods we established the bioburden and identified the microorganisms that were isolated from papery, books and storage environment. These microorganisms were subjected to analise and we tested their resistence to gamma radiations. It is known that the ionizing radiations induce the denaturation or cleavage of nucleic acids in all living organisms present on and inside the treated material, but at a short time of exposure is ineficient because a high number of bacteria and fungi present a mechanism of DNA reparations.
The first radiobiological tests that we had to carried out is to test the efficiency of the treatment against microorganisms. This step is necessary because it is known that a high dose of radiation could induce damage by modification of the cellulose and its degree of polymerization making it more fragile and brown-colored.
This work represents a part of a complex study for establishing the methodology treatment of archives developed in the form of national project (ARCON 93-086). The samples were provided by IFIN-HH archives and .Muzeul Brailei. museum.
P30
Binary sensitive systems based on A-ProOMe/AAc synthesized by gamma radiation
G. González-Pérez, S. Castillejos, G. Burillo,,Departamento de Química de Radiaciones y Radioquímica, Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, México DF 04510, México
Thermo and pH-responsive gels have received much interest in different fields of knowledge for their applications in drug delivery systems, controlled release systems for targeted delivery to specific areas of body and others. Poly(acryloyl-L-proline methyl ester) (A-ProOMe) exhibits a lower critical temperature (LCST) of 14oC and polyacrylic acid (PAAc) exhibits a critical pH response at about 4.5 °C. In this work, three different systems containing (A-ProOMe) and (PAAc) were synthesized by gamma radiation; a) A-ProOMe grafted onto PAAc hydrogel .comb type hydrogel.; b) radiation binary grafting of A-ProOMe and AAc onto PP by mutual irradiation method PP-g-AAc/A-ProOMe; and c) and binary grafting of those monomers in two step radiation method (PP-g-AAc)-g-(A-ProOMe). The characterization of the graft copolymers was examined by infrared (FTIR-ATR), and thermal analysis (TGA). Limit swelling behavior, pH sensitivity and Cu+2 uptake of different systems will be discussed.
The authors wish to express their thanks to S. Castillo-Rojas, B. Leal, F. García, and M. Cruz, from ICN-UNAM, for technical assistance. This work was supported by DGAPA-UNAM Mexico, Grant IN200108.
P31
Oxidation mechanism of oligomer model compounds for polymer electrolyte fuel cell membranes
S. M. Dockheer, W. H. Koppenol, Institute of Inorganic Chemistry, Swiss Federal Institute of Technology,
8093 Zurich, Switzerland
L. Gubler, Electrochemistry Laboratory, Paul Scherrer Institut, 5232 Villigen PSI
Reactions of hydroxyl radicals with sulfonated aromatics are of interest because of their presumed involvement in the degradation of fuel cell membranes [1]. There has been a renewed interest in sulfonated aromatics as a cheaper and environmentally friendly alternative to the widely used perfluorocarbon materials. However, membrane durability has become a central issue for the progress of PEFC technology. On the basis of pulse radiolysis experiments with an oligomer of poly(sodium styrenesulfonate) and poly(sodium α-methylstyrenesulfonate), we propose a detailed mechanism that starts with hydroxyl radical addition to the aromatic ring and concomitant benzylic hydrogen abstraction, followed by the reversible addition of dioxygen to the benzylic radical. We investigate conditions that favour formation of ROOH in the polymer backbone. Recently, it has been shown that introduction of the co-monomer metacrylonitrile into the membrane resulted in a notable improvement of the lifetime of the membrane [2]. Therefore, we present comparison studies with nitrile model compounds to elucidate the stabilizing effect of the nitrile group. In combination with standard electrode potentials of couples with relevance for the fuel cell membrane, we propose a chain scission mechanism. The knowledge of mechanistic details might serve as a basis for further membrane improvement.
References:
1. H. Liu et al.: Polymer Electrolyte Fuel Cell Durability, Springer Science and Business Media, 71 (2009)
2. H. B. Youcef et al: Electrochemistry Communications 11, 941 (2009)
P32
Scavengers in macromolecular crystallography: do they help?
E. F. Garman,1 E. de la Mora1,2, 1Laboratory of Molecular Biophysics, Biochemistry Department, Oxford University, South Parks Road, Oxford, OX1 3QU, U.K. 2Departamento de Medicina Molecular y Bioprocesos, Instituto de Biotecnologia,Universidad Nacional Autonoma de Mexico, P. O. Box 510-3 Cuernavaca, Mor 62271,Mexico
I. Carmichael, Notre Dame Radiation Laboratory, University of Notre Dame, Notre Dame, IN 46556, USA
Radiation damage continues to present a problem to macromolecular crystallographers using cryo-cooled protein crystals at synchrotrons where a linear decay in diffraction intensity is observed with increasing dose [1]. Free radical scavengers and radioprotectants have been suggested as a possible means of reducing the rate of this damage. Early room temperature (RT) experiments seemed to show that styrene and PEG might have a positive effect on the dose tolerance of crystals, but the idea was not systematically pursued. We have previously reported that 0.5 M-1 M ascorbate incorporated by cocrystallisation was effective in quenching the disulphide breakage in lysozyme (HEWL) crystals during 100 K data collection [2]. The screening of a large number of potential radioprotectants was then undertaken with an on-line microspectrophotometer using cystine and cysteine respectively to model protein disulphide bonds and thiol groups, and observe any quenching of the disulphide anion peak. Evidence for the potential of ascorbate as a radioprotectant was strengthened, and 1,4 benzoquinone, 2,2,6,6-tetramethyl-4-piperidone (TEMP) and reduced dithiothreitol also showed promise [3].
In recent work [4] to search for RT radiation damage mitigation strategies, three of these putative radioprotectants were tested. The results indicate that ascorbate and 1,4-benzoquinone are effective radioprotectants, whereas studies on TEMP were inconclusive. Ascorbate offered a 2´ enhancement of crystal dose tolerance, whereas benzoquinone gave a >8´ increase at the dose-rates used. The universally previously observed exponential form of the RT diffraction intensity decay was modified by the addition of scavengers to become linear as is observed at 100 K without scavengers present. The radiation damage mechanisms are elucidated by these results, which enable postulates to be made on the radical species causing damage at 100 K. Recent results using the electron scavenger, sodium nitrate, soaked into HEWL crystals will be discussed.
References:
[1] R. G. B. Ravelli, E. F. Garman: Current Opinion of Structural Biology 16, 624 (2006)
[2] J. M. Murray, E. F. Garman: J. Synchr. Radiat. 9, 347 (2002)
[3] R. J. Southworth-Davies, E. F. Garman: J. Synchr. Radiat. 14, 73 (2007)
[4] A. I. Barker, et al.: J. Synchr. Radiat. 16, 205 (2009)
P33
Modification of the water absorbance of cellulose by radiation grafting
E. Takács, L. Wojnárovits, Institute of Isotopes, Hungarian Academy of Sciences, Budapest, Hungary
J. Borsa, Budapest University of Technology and Economics, Budapest, Hungary
The use of natural cellulose fibres as reinforcing elements in macromolecular composite materials has recently gained considerable attention, as emphasized by the numerous articles on the topic [1-4]. However, the preparation of cellulose-based composites is perturbed by the highly hydrophilic character of the fibres, which is associated with a low interfacial compatibility with hydrophobic polymeric matrices, as well as with a loss of mechanical properties after moisture uptake. In order to reduce the hydrophilic character of cellulose fibres and to improve the strength of their adhesion to the matrix, it is necessary to undertake a structural modification of their surface. Irradiation induced grafting of hydrophobic polymer chains onto the surface of cellulose fibres is a convenient technique for this purpose.
In this work 2-ethylhexyl acrylate and 2-ethylhexyl methacrylate monomers were grafted onto cotton cellulose. The grafting yield was measured by the percent increase in the mass of the samples and also by FTIR spectroscopy. Optimum grafting conditions were determined by varying the monomer concentration, grafting time, irradiation temperature, and dose. The grafting was performed using both the preirradiation and mutual grafting methods.
SEM pictures clearly showed the formation of a coating layer on the fibres. The swelling in water decreased with increasing grafting yield indicating the reduction of the hydrophilic character of the samples. Polymer compatibility of the grafted samples was checked by preparing polymer composite samples.
References:
[1] M. N. Belgacem, A. Gandini: Composite Interfaces, 12, 41 (2005)
[2] E. Takács at al.: Nucl. Instr. Meth. Phys. Res., B, 236, 259 (2005)
[3] N. Benke, E. Takács, L. Wojnárovits, J. Borsa: Radiat. Phys. Chem. 76, 1355 (2007)
[4] E. Takács et al.: Nucl. Instr. Meth. Phys. Res., B, 265, 217 (2007)
P34
Characterization of crosslinking and chain scission on irradiated ETFE
A. B. Lugao, D. F. Parra, H. F. Araujo, A. N. Geraldes, H. Zen, G. Ribeiro
Institute for Energy and Nuclear Research, 2242 Sao Paulo, SP 05508-000, Brazil
Fluorinated polymers are known to be prone to degradation under radiation. Crosslinking under melt conditions were developed in the last few years [1, 2], it occurs in absence of oxygen at just above melting temperature of PTFE. The crosslinks are formed through Y-type structure. Crosslinked PTFE has been attained by irradiation in its molten state above the melting temperature under oxygen-free atmosphere. It shows improvements in radiation resistance [3], mechanical properties, friction, light transparency, compared to non-crosslinked PTFE [4]. Another recent process to promote PTFE crosslinking was based on PTFE irradiation in acetylene [5].
Ethylene-tetrafluoroethylene copolymer (ETFE) is a thermoplastic partially fluorinated polymer with many important technological application in wire and cable industry. It has been crosslinked by high energy in presence of multifunctional agents. Recently a potential application of styrene grafted membranes has fuelled the interest in ETFE crosslinking and many studies were conducted by Scherer.s group at PSI [6]. Perfluorinated polymers are insoluble. Ethylene-tetrafluoroethylene copolymer is also difficult to dissolve; therefore its crosslinking behaviour is hard to study. Fortunately, crosslinking changes the mechanical behaviour of polymers, but this evaluation is also hard as the mechanical properties of partially crystalline polymers are dominated by morphology. One way to evaluate crosslinking is examining the properties under melting.
References:
[1] Y. Tabata: Proceedings of Taniguchi Conference. Sapporo. 118, (1992)
[2] S. Jiazhen, Y. Yuefang, Y. Xiaoauana, Y. Wanxi: Radiat. Phys. Chem. 42, 139 (1993)
[3] Y. Tabata, A. Oshima, K. Takashika, T. Seguchi: Radiat. Phys. Chem. 48, 563 (1996)
[4] Y. Tabata, A. Oshima: Macromolecular Symposia, 143, 337 (1999)
[5] N. M. Bol.bit et al.: High Energy Chemistry, 42, 354 (2008)
[6] A. Gursel et al.: Journal of Membrane Science, 311, 208
P35
Production of HCl and H2 in the radiolysis of PVC
P. I. Pavlova-Schmitz, S. Yeates, School of Chemistry, University of Manchester, UK
J. A. LaVerne, Radiation Laboratory and Department of Physics, University of Notre Dame, USA
S. M. Pimblott, School of Chemistry and Dalton Nuclear Institute, University of Manchester, UK
PVC is a thermoplastic polymer commonly encountered in nuclear waste management, disposition and disposal. The effects of ionising radiation on its performance are insufficiently understood. Our study has focused on the post-irradiation degradation of γ and 4He ion irradiated PVC and on the release of potentially corrosive and explosive gases, such as HCl and H2, from deaerated, aerated, and water mixtures of PVC.
Experiments were performed with PVC powder samples with number average weight of 22000 D and with unplasticised PVC film. The samples were irradiated with 60Co γ-rays and 4He ions at room temperature. The production and post-irradiation release of HCl and H2 was measured using ion and gas chromatography, respectively. The post-irradiation evolution of the solid plastic was examined using GPC and EPR, FT-IR and UV/VIS spectroscopy. UV/VIS absorbance spectroscopy was performed on PVC powder dissolved in THF. FT-IR transmittance spectra were obtained from PVC powder in air. EPR spectra were taken after γ-irradiation of PVC powders in air and vacuum, and for PVC film irradiated with γ-rays and 4He ions. GPC measurements were made on both pure and irradiated PVC powder and PVC film.
The yield of HCl from γ-radiolysis of PVC powder is 19.6 molecules/100 eV. Chloride ion is released from the polymer for days following radiolysis. The H2 yield with γ-rays is about 0.23 molecule/100 eV and 0.45 molecule/100 eV with 4He ion radiolysis. Visually, irradiated PVC samples change from white to a green-brown colour with the extent of discolouration dependent on the applied dose and irradiation environment. No significant post-irradiation evolution of the chromophores was observed by FT-IR or UV/VIS, but the EPR spectra obtained evolve with time. GPC analysis showed a molecular weight distribution with a smaller mean in the irradiated samples compared to pure PVC. This change is related to HCl loss and possibly to main chain scission (fragmentation). Various reaction mechanisms and possible products will be discussed.
This work was supported by the UK Nuclear Decommissioning Authority and by the Office of Basic Energy Science of the US Department of Energy.
P36
Radiation degradation and stability of polyurethanes
M. Walo, G. Przybytniak, Centre for Radiation Research and Technology, Institute of Nuclear Chemistry and Technology, Warsaw, Poland
Polymeric biomaterials might be exposed to ionising radiation in order to improve their properties or to carry out sterilization that may also contribute in changes of the macroscopic features. It is generally known that irradiation affects physicochemical and mechanical properties of polymers leading usually to crosslinking and/or degradation. Crosslinking transforms a linear polymer into a tree dimensional network in resulting improvement of mechanical properties. The decrease in molecular weight is a consequence of degradation and leads to deterioration of polymer properties. A proportion between both processes determines final effect.
Polyurethenes are a well-known class of polymers with the characteristic .NH-CO-O- linkage in the chain. They are block copolymers, which physicochemical properties may be modified by changing the ratio between soft and hard segments. Type and proportions between segments determine the character of polymer and consequently their industrial utility. Polyurethanes are known as radiation stable materials but their behaviour upon the exposure to ionising radiation is strongly dependent on their chemical and physical structure.
Segmented polyurethanes have found application in medicine as biomaterials due to good biocompatibility, hydrolytic and oxidative biostability, excellent mechanical properties and good processibility. They are used for production of scaffolds in tissue engineering and for manufacturing medical devices, such as vascular grafts, artificial hearts, catheters and mammary implants.
The aim of this study was to characterize influence of ionizing radiation on the selected physicochemical properties of polyurethanes. Samples of polyurethanes were investigated before and after irradiation by EPR, DSC, TG, gas chromatography, measurement of dynamic contact angle and mechanical properties. On a basis of the study we found that radiation sterilization has not significant influence on physicochemical properties of segmented polyurethanes and two parallel, competitive processes: crosslinking and chain scisson were observed.
P37
Radiation preparation of small sized zinc oxide particles doped by Ga(III) and Ln(III) ions
T. Gbur1, V. Čuba1, V. Múčka1, M. Pospí.il1, M. Nikl2, 1 Czech Technical University in Prague, Faculty of Nuclear Sciences and Physical Engineering, Department of Nuclear Chemistry, Břehová 7, 115 19 Praha 1, Czech Republic 2 Institute of Physics of the AS CR, v. v. i., Na Slovance 2, 182 21 Praha 8, Czech Rrepublic
Radiation preparation of zinc oxide nanopowder from aqueous solutions via accelerated electrons and gamma irradiation was studied. Generally, exciton luminescence of zinc oxide is conveniently placed on the border of ultraviolet and visible areas, at 395 nm. Furthermore, when doped with elements from III. group or lanthanide series it has significantly sub-nanosecond lifetime [1, 2], which makes it very suitable for possible application as scintillation material with superfast response.
For the formation of ZnO particles, as well as for the subsequent degradation of additional compounds, radiolysis of water plays crucial role. The probable mechanisms of oxide nanoparticles formation is partial or total radiation reduction of zinc(II) ions to metallic particles via reactions with hydrated electrons
Zn2+ + 2e-aq → Zn0
followed by their oxidation via reaction with oxygen under suitable conditions [3]:
2Zn + O2 → 2ZnO
In performed experiments LINAC-4-1200 generating 4.5 MeV electrons and 60Co γ-rays were employed as sources of radiation. Solid phase was prepared by irradiation of aqueous solutions containing zinc(II) ions, propan-2-ol, polyvinylalcohol, and hydrogen peroxide. For study of formation of ZnO doped with Ga(III) or Ln(III) ions, low concentrations of gallium nitrate or lanthanum acetate were added. Optimum dose of irradiation was estimated to be 70 kGy. Various physicochemical parameters, including scintillation properties of prepared materials, were studied. Crystalline zinc oxide was found in solid phase either directly after irradiation, or after heat treatment. Size of the particles ranged from 50 to 150 nm. After decomposition of impurities and annealing of oxygen deficiencies, the samples showed intensive emission in visible range and well-shaped exciton luminescence at 390-400 nm. Irradiation with accelerated electrons was more effective than gamma irradiation.
References:
[1] S. E. Derenzo, M. J. Weber, M. K. Klintenberg,: Instr. Meth. Phys. Res. A., 486, 214 (2002)
[2] J. S. Neal et al.: Nucl. Instr. Meth. Phys. Res. A., 568, 803 (2006)
[3] F. Ren et al.: J. Phys. D: Appl. Phys., 39, 488 (2006)
P38
Radiation grafting of 4-vinylpyridine onto PP-g-AAc hydrogel films, and its copper (II) complexes
L. M. Lazo1, G. Burillo2, A. Solano3, 1Posgrado de Ingeniería, Facultad de Química, Universidad Nacional Autónoma de México, 2Instituto de Ciencias Nucleares. Universidad Nacional Autónoma de México, 3Facultad de Química, Universidad Nacional Autónoma de México, México
A new polymeric system consisting of a comb type copolymer of 4VP onto PAAc hydrogel, grafted onto PP film net-(PP-g-AAc)-g-4VP, was synthesized by gamma radiation in three consecutive steps. This polymeric system has better mechanical properties that the studied hydrogels, pH sensitivity and the ability to form complex with transition metal ions. The synthesized system has chelating ability with transition metal ions due to its structure containing nitrogen and oxygen atoms. In this work we present the swelling behavior of the supported comb-type hydrogel and its complexes with copper (II) ions in acidic aqueous medium. These systems were studied by IR, UV-Vis and EPR spectroscopy. Two critical pH values were found for net-(PP-g-AAc)-g-4VP at 4.5 and 7.2. Adsorption capacity of Cu(II) ions by the system is 40 mg/g dry polymer, the capacity increases with the increase of pH. The FTIR spectrum of the supported comb-type hydrogel-copper (II) complex shows the appearance of carboxilate groups due to the ion exchange reaction between the supported comb-type hydrogel and Cu(II) ions. The results suggest pH dependence and a coordination mechanism through carboxylate groups of acrylic acid and the nitrogen atom of the pyridine groups of the supported comb-type hydrogel is propose.
The chelating ability and pH-responsive behavior of synthesized system make it an attractive material for possible applications in adsorption, enrichment and separation processes from low level wastewater.
The authors are grateful to PAPIIT-UNAM, IN200108; CONACyT and Posgrado de Ingeniería, Facultad de Química, for support and to S. Castillo, F. García and B. Leal from the ICN-UNAM, for technical assistance.
P39
Radiation-induced grafting of thermo and pH sensitive polymers
E. Bucio, Departamento de Química de Radiaciones y Radioquímica, Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, 04510, México D.F., Mexico
Radiation processing has many advantages over other conventional methods. When using radiation for material processing, no catalysts or additives are needed in order to initiate the reaction. Polymeric films were modified by gamma-radiation grafting of pH and thermo sensitive monomers by one and two step methods, using the pre-irradiation and the direct methods. The effects of the absorbed dose, monomer concentration and reaction time were investigated. The surface chemistry of grafted samples was analyzed by FTIR-ATR spectroscopy, while their thermal properties were analyzed by TGA and DSC. The stimuli-responsive behavior was studied by swelling and contact angle in water, as well as by DSC. Sensitive films presented a critical pH point and low critical solution temperature. Temperature and pH stimuli-responsive macromolecular materials have attracted great attention because of their obvious applications in biomedicine and biotechnology.
This work was supported by DGAPA-UNAM Grant IN200208.
P40
Preparation of copper and cuprous oxide nanoparticles by radiation-induced reduction of copper ions
J. Bárta, M. Pospí.il, V. Čuba, Czech Technical University in Prague, Faculty of Nuclear Sciences and Physical Engineering, Břehová 7, 115 19 Prague, Czech Republic
Nanometre-sized metallic materials have drawn much attention because of their interesting properties, namely large specific surface area and related catalytic activity. A promising preparation methods is radiation-induced reduction [1]. The advantages of this method are the possibility to form particles with uniform size distribution at room temperature. Often OH scavenger must be added prior to irradiation in order to prefer reductive processes. Many non-noble metal particles in aqueous solutions are readily oxidised by dissolved oxygen.
In this work, aqueous solutions containing copper sulphate (10-3 to 10-2 mol.dm-3) in the presence of propan-2-ol (10 % v/v) and various surfactants . polyvinyl alcohol PVA, sodium hexametaphosphate SHMP and sodium alginate . were used without pH adjustment. These solutions were deaerated by N2 for one hour and then irradiated by accelerated electrons of 4.5 MeV mean energy. Optical absorption spectra measurement was carried out by Varian Cary 100 UV-VIS spectrophotometer; pH was measured and solid phase was separated in the glove-box under nitrogen using ultrafiltration cell. Solid phase was characterised by XRPD and SEM.
After irradiation (dose 10 kGy), solutions containing both ∙OH scavenger and any of studied surfactants changed colour to dark violet or pink, which corresponds with measured absorption spectra, showing broad band in the 300 . 500 nm range and copper surface plasmon peak at c.a. 580 nm. Both effects rapidly disappeared in the presence of air as a result of copper particles oxidation. According to expectations, the pH decreased during irradiation from natural pH c.a. 5.6 3.3. The kinetics of the oxidation was studied with respect to changes of pH and optical absorption spectrum. Whereas the pH steadily increased, the absorbance changed non-monotonously . in the first stage, a rapid increase of absorbance in the range 600 900 nm occurred, followed by long lag-phase and finally in the third stage, copper absorption spectrum reappeared, but slowly diminished.
In all separated brown or black solid phases, XRPD analysis identified crystalline copper, cuprous oxide or their mixture. According to literature [2], the product determining parameter appears to be pH (at low pH, copper is preferred). SEM micrographs showed that prepared particles are spherical in shape and have high size range (from c.a. 30 nm up to 400 nm in diameter, average size being c.a. 200 nm).
References:
[1] J. Belloni et al.: New J. Chem., 1239 (1998)
[2] H. Ping, S. Xinghai, G. Hongcheng: J. Colloid Interface Sci. 284, 510 (2005)
P41
Radiation preparation of nanosized nickel oxide catalysts
T. Pavelková, V. Čuba, M. Pospí.il, V. Múčka, CTU in Prague, FNSPE, Břehová 7,
115 19 Prague 1, Czech Republic
The aim of this study was to prepare nickel oxide nanoparticles obtained from aqueous solutions using radiation energy. While works dealing with radiation preparation of nickel nanoparticles are quite numerous [1, 2], reports dealing with radiation preparation of nickel oxide are rather scarce.
Properties of NiO and other catalysts are affected by the method of preparation. It varies the purity, the size of crystallites, the stoichiometry, the specific surface area, the surplus oxygen, and the amount of active catalytic centres. Particle size uniformity and high chemical purity of prepared materials are advantages of radiation method.
Five basic aqueous solutions containing nickel formate and other compounds, including hydrogen peroxide, polyvinyl alcohol (PVA), and isopropyl alcohol (IPA), were used for preparation of nickel oxide. Prepared solutions were irradiated by accelerated electrons with doses in the range 0 ― 200 kGy. During irradiation, the solutions changed their colour due to the formation of solid phase. In few cases, the formation of true colloid was observed. With increasing dose up to 80 kGy, the yield of the solid phase increases.
Changes caused by irradiation were determined by UV/VIS spectrophotometry in the range of 190-900 nm. The solid phase from the solutions irradiated with the optimum dose of 80 kGy was separated via ultrafiltration and carefully dried. The composition of the solid phase was determined via X-ray powder diffraction. From diffractograms the size of crystallites was determined. The specific surface area was measured using isothermal adsorption of nitrogen from gas mixture hydrogen . nitrogen. Catalytic activity was studied by catalytic degradation of aqueous solution of hydrogen peroxide. Rate constants were measured at four temperatures. The solid phase was annealed under vacuum at 200 °C for 2 hours. The characteristics of annealed and unannealed materials were compared using all previously mentioned methods.
The solid phase formed was found to be pure non-stoichiometric nickel oxide in all studied solutions; the size of the particles was calculated to be in order of tenth of nm. The catalytic activity of samples was determined to be significantly higher compared to commercial non-stoichiometric NiO (LACHEMA).
The obtained results indicate that radiation technique is a viable and very promising method for preparation of highly pure NiO catalysts with uniform small-sized particles.
References:
[1] S. V. Gornostaeva et al.: Protection of Metals, 44, 372 (2008)
[2] M. Pospí.il et al.: Radiat. Phys. Chem., 77, 968 (2008)
P42
Biradical formation in the radiolysis of cycloalkanes
R. H. Schuler, Radiation Laboratory, University of Notre Dame, Indiana, USA
L. Wojnarovits, Institute of Isotopes, Hungarian Academy of Sciences, Budapest, Hungary
In the radiolysis1,2 and vacuum-ultraviolet photolysis3 of liquid cycloalkanes structural isomerisations to open-chain olefins, while in the cases of 1,2-, 1,3- and 1,4-dimethylcycloalkanes also geometrical (cis«trans) isomerizations were observed. Biradicals are suggested as intermediates of these isomerizations.
Iodine scavenging studies, similar to that previously performed for n-alkanes and isoalkanes,4,5 were made in the g radiolysis of cyclopentane, cyclohexane, cycloheptane, cyclooctane and cyclodecane. Using gel permeation chromatography (GPC) for the separation of iodide scavenging products a,w-diiodo alkanes were also found to form in addition to the usual scavenging products, cycloalkyl iodides and fragment iodoalkanes. The production of a,w-diiodo alkanes was attributed to biradical scavenging. The diiodo alkane yields showed a strong dependence on iodine concentration revealing a Stern-Volmer type competition between unimolecular stabilization of the biradicals forming hydrocarbon products and the radical scavenging reaction. This competition allowed to estimate the biradical lifetimes, 100-600 ns, and the biradical yields, G = 0.06 . 0.25 biradical/100 eV.
References:
1. G. R. Freeman, E. D Stover: Can. J. Chem. 46, 3235 (1968)
2. L. Wojnarovits, G. Foldiak: Acta Chim. Acad. Sci. Hung. 93, 1 (1977)
3. L. Wojnarovits, L. Kozari, Cs. Keszei, G. Foldiak: G. J. Photochem. 19, 79 (1982)
4. L. Wojnarovits, R. H. Schuler: J. Phys. Chem., A., 104, 1346 (2000)
5. R. H. Schuler, L. Wojnarovits: L. J. Phys. Chem., A., 107, 9240 (2003)
P43
Preliminary study of gamma irradiation induced chemical, mechanical and optical changes
in some sorts of paper
M. Virgolici, M. M. Manea, I. V. Moise, D. C. Negut, R. Suvaila, M. Cutrubinis, R. Georgescu, IRASM Irradiation Technology Center, .Horia Hulubei. National Institute for Physics and Nuclear Engineering,
407 Atomistilor Str., 077125, Magurele, Ilfov County, Romania
A. V. Medvedovici, Department of Analytical Chemistry, Faculty of Chemistry, University of Bucharest,
90-92 Panduri Str., 050663, Bucharest, Romania
I. Stanculescu, Department of Physical Chemistry, Faculty of Chemistry, University of Bucharest,
4-12 Bd. Regina Elisabeta, 030018 Bucharest, Romania
C. Stanciu, C. M. Talasman, Developement and Research Institute for Pulp and Paper CEPROHART S.A.,
3, Al.I.Cuza Blvd., Braila 6100, Romania
Present study focuses on the characterisation of some sorts of gamma irradiated cellulose based materials with a wide dose range that covers most of the technological irradiation applications including archives and mail decontamination. Volatile radiolysis products, thermal stability, chemical structure, colour and mechanical properties were investigated in correlation with the absorbed dose and free radicals concentration.
Volatile radiolysis products were determined by means of dynamic headspace / thermal desorption of air surrounding the samples enclosed in sealed containers and supplementary by direct thermal desorption of small sheets of paper. Markes Unity thermal desorber was coupled with an Agilent GC 6890N gas chromatograph and an Agilent 5975 inert EI mass spectrometric detector for semi-quantitative evaluations and structural characterization by means of retention index and mass spectrum matching with commercial libraries. GC/MS screening was performed in electron ionisation mode, scanning from 10 to 701 amu. AMDIS software was used for experimental mass spectra deconvolution for improved spectral matching. Thermal stability was investigated by dynamic thermogravimetry and dynamic scanning calorimetry using a Netzsch STA 409 PC simultaneous thermal analyzer. Thermogravimetric information was used for direct thermal desorbtion temperatures optimization, determination of the percent of inorganic fillers from paper materials and further correlations with the cellulose molecular structure and physical properties. Macromolecular structure characterization was performed by Fourrier Transformed Infrared and Raman spectroscopy using a Bruker Vertex 70 FT-IR spectrometer equipped with a RAM II Raman module. FT-IR was performed in transmission mode with KBr pellets and FT-Raman with a Nd:YAG laser excitation source of 1064 nm. From the FT-IR spectra were calculated the crystallinity factor, hydrogen bonding and the oxidation index of cellulose for correlations with the degree of molecular structure degradation. Mechanical properties and colour changes were monitored for further correlation with structural modifications induced by gamma irradiation and ageing, using Zwick/Roell Z005 and INSTRON universal testing machines and the Hunterlab Miniscan XE digital colorimeter. The depletion of the free radicals trapped in samples after irradiation was determined with a Magnettech MiniScope MS200 electron spin resonance spectrometer.
P44
Radiation-induced electron paramagnetic resonance signal and soybean isoflavones content
M. R. R. de Oliveira1, J. M. G. Mandarino2, N. L. del Mastro1, 1Nuclear and Energy Research Institute,
IPEN-CNEN/SP, S.o Paulo, SP, Brazil, 2EMBRAPA, Londrina, PR, Brazil
Flavonoids are found in all higher plants, and many display strong absorption in the UVB spectral region. It has been suggested that they play a role in the protection of plants by screening vital cellular components from damaging UV radiation. The isoflavones from soybean are known to have a number of beneficial health effects and can act as antioxidant i.e. quench oxidation by transferring hydrogen atoms to free radicals. In food, free radicals can be generated by several commonly used industrial processes, such as radiosterilization or heat treatment. EPR spectroscopy has been used to detect radioinduced free radicals in food. Upon gamma-ray irradiation, a new ESR signal was detectable in the vicinity of g = 2.0 region, ascribed to stable cellulose-derivative components and is a good indicator in the identification of irradiated plant samples. In this work the relation between EPR signal induced by gamma irradiation treatment and soybean isoflavones content was investigated. Present results did not show correlation between total isoflavones content and the EPR signal. Nevertheless, some isoflavone contents had a negative correlation with the radiation-induced EPR signal.
P45
Electrochemical behaviour of stainless steel under irradiation at high temperature and high pressure
B. Muzeau, CEA/DSM/IRAMIS/LSI . UMR 7642 . Ecole Polytechnique
S. Perrin, CEA/DEN/DANS/DPC/SCCME/LECA . CEA / Saclay
C. Corbel, CEA/DSM/IRAMIS/LSI . UMR 7642 . Ecole Polytechnique
D. Simon, CNRS/CEMHTI . UPR3079 . CNRS / Orléans
D. Féron, CEA/DEN/DANS/DPC/SCCME. CEA / Saclay, France
In the primary coolant circuit of pressurized water reactors (PWR), water radiolysis occurs at high temperature (HT), 280-320 °C, and high pressure (HP), 15.5 MPa. Few data are available in the literature on the role of HT-HP water radiolysis on the corrosion of metallic reactor components.
The present approach uses an high energy ion beam to control the production of radiolytic species at the AISI316L/water interface in a HP-HT electrochemical cell working in the range [25 °C, 0.1 MPa] - [300 °C, 9 MPa]. The cell is designed to record the free potential of the AISI 316L/water interface mounted in line on the CEMHTI (CNRS-Orléans) cyclotron delivering the ion beam. The free potential evolution is compared before, during and after irradiation.
Results are reported where an aqueous solution ([B] = 1000 ppm, [Li] = 2 ppm, [H2(aq)] = 4.1x10-5 mol L-1 at 25 °C) is used to model the PWR primary coolant circuit. The AISI 316L/water interfaces are irradiated at 300 °C with high energy protons emerging at ~22 MeV at the interface with a flux varying by four orders of magnitude, from 107 to 1011 H+,cm-2,s-1. The free potential evolution under irradiation has been recorded and seems to depend on the flux, but also on the ageing of the AISI 316L target at 300 °C and/or under irradiation. The behavior of the free potential under irradiation is highly dependent on temperature and pressure.
During the experiment, an hydrogen permeation probe allows the measurement of the hydogen amount in the solution, introduced initially or produced through the radiolysis. It enables to investigate in situ the interaction between a major product of water radiolysis, the hydrogen, and an irradiated solid/liquid interface.
P46
Pulse radiolysis of p-terphenyl in selected ionic liquid
R. Kocia, J. Grodkowski, J. Mirkowski, Centre for Radiation Research and Technology,
Institute of Nuclear Chemistry and Technology, Dorodna 16, 03-195 Warsaw, Poland
Room temperature ionic liquids (ILs) are regarded as low volatility and combustion-resistans solvents. They serve as good media for various reactions and have been suggested as green solvents in many applications. Their specific properties and promising experiments regarding applicable of IL in nuclear industry brought the question of radiation chemistry of IL.
In the present study the properties of intermediates derived from p-terphenyl (TP) in the methyltributylammonium bis-[(trifluoromethyl)sulfonyl]imide (R4NNTf2) solutions have been examined by pulse radiolysis. TP intermediates (anionic, cationic and excited species) have absorption spectra in UV/VIS range. The pulse radiolysis of TP solution in R4NNTf2 gives afterwards some insight into the nature of primary products of R4NNTf2 radiolysis.
TP photocatalytic activity was the main reason to select this compound for experiments in IL. p-Terphenyl anion radical (TP·-) (often used in carbon dioxide reduction) is created photochemically chiefly from singlet excited states 1TP* by reaction with electron donor triethylamine (TEA) and in radiolysis directly in reaction with solvated electrons. TP* in IL solution can also be formed by energy transfer from excited radiolysis products of IL and in direct TP excitation by Čerenkov light.
To discern between different TP derived intermediates and characterize primary radiolysis products of R4NNTf2, the pulse radiolysis of TP solution has been carried out under appropriate gases such as Ar, CO2, O2 and N2O in the presence or absence of TEA. As sensors of excited state formation benzophenone (BP) TP have been selected.
Fast kinetic measurements have been carried out using 10 ns, 10 MeV, electron pulses from a LAE 10 linear electron accelerator delivering the dose up to 20 Gy per pulse.
P47
Radiolysis behavior of ionic liquids of thiocyanate salts
R. Nagaishi, N. Aoyagi, Nuclear Science and Engineering Directorate, Japan Atomic Energy Agency
M. Taguchi, Quantum Beam Science Directorate, Japan Atomic Energy Agency
T. Kondoh, J. Yang, Y. Yoshida, The Institute of Scientific and Industrial Research, Osaka University, Japan
Hydration and reactions of Eu(III) ion [1] and electron [2] in hydrophobic ionic liquids (IL) by adding a small amount of water have been studied by using the laser-induced fluorescence spectroscopy and pulse radiolysis. In the radiolysis of ILs, the ionization sites and the presence of geminate radical formed together with electron are not clear. The radiolytic reactions in hydrophilic ILs containing thiocyanate ion as a constituent, which reacts with OH in aqueous solutions, were studied to understand the formation and reaction of products in ILs.
ILs were synthesized by the combination of several anions and cations in acetone or water: thiocyanate bromide (BrSCN) was used as anion source, while lithium bis(trifluoromethanesulfonyl)imide (Litfsi = LiN(SO2CF3)2) was used for the comparison; chlorides of imidazolium (C4mim+: 1-butyl-3-methylimidazolium, C2mim+: 1-ethyl-3-methyl imidazolium) and ammonium (demma+: N,N-diethyl-N-methyl-N-(2-methoxyethyl)ammonium) were mainly used as cation sources. The samples were irradiated by electron pulses (28 MeV, 8 ns) from L-band LINAC at ISIR, Osaka Univ. to observe transient absorption of electrons and radicals formed in the samples.
In the imidazolium salt of [C4mim][tfsi], the transient absorption originated from C4mim+ was observed, while neither electron nor radical originated from tfsi- observed. On the other hand, the absorption ascribed to dimer radical anion of (SCN)2- was observed in [C4mim][SCN] as well as that from C4mim+. The rapid formation of (SCN)2-, irrespective of the high viscosity of ILs, could be explained by the direct dimerization between SCN radical formed in ILs and its surrounding SCN-, not via their diffusive encounter. In order to elucidate the formation yield and pathway of SCN radical, the ammonium salts containing two anions, i.e., the mixtures of [demma][SCN] and [demma][tfsi] were prepared. The initial absorbance of (SCN)2- increased with increasing the fraction of [demma][SCN] in the mixed ILs, where any radicals originated from demma+ were not observed. This suggests that one of the ionization sites of ILs is SCN- to generate a pair of electron and SCN radical.
References:
[1] R. Nagaishi et al.: J. Alloys Comp., 431, 221 (2007)
[2] A. Asano et al.: Radiat. Phys. Chem., 77, 1244 (2008)
P48
Pulse radiolysis study of the oxidizing radicals in ionic liquid
M. Nyga1, J. Grodkowski1, T. Szreder1,2, J. Mirkowski1, 1Centre of Radiation Research and Technology, Institute of Nuclear Chemistry and Technology, Dorodna 16, 03-195 Warsaw, Poland, 2Faculty of Biotechnology and Food Sciences Technical University of Łódź, Poland
Ionic liquids may provide a solution to many technological problems. Their properties, negligible vapor pressure, non-flammability and ability to be reused made them attractive alternatives to classical solvents. Our understanding of this new class of solvents is quite poor in some areas. The radiation chemistry of ILs is one of them. Recently absorption spectra of Br2.-, (SCN)2.- and BrSCN.- was observed.
The rate constants of several elementary reactions of the intermediate formation in the solution of dibromoethane, SCN- and Br- in methyltributylammonium bis[(trifluoromethyl)sulfonyl] imide R4NNTf2 were studied by ns pulse radiolysis. The rate constants of the reactions between Br2.- and SCN- are of the same order of magnitude as for Br2.- with chloropromazine in R4NNTf2 [1]. The energy of Br. solvation is the driving force of Br2.- formation.
At this time the formation of Br2.- radical anions with azide anions were studied by pulse radiolysis.
Br2. - + N3- → 2 Br- + N3.
We demonstrated that N6.- can be generated in ionic liquids methyltributylammonium bis[(trifluoromethyl)sulfonyl] imide R4 NNtf2 according to a reaction.
N3. + N3- D N6.-
It reveals board absorption band in the range of 650 . 700 nm. Alfassi et al. [2] have shown that N6.- absorb at 650 nm with extinction coefficient of 8000 M-1cm-1.
References:
[1] J. Grodkowski; P. Neta: J. Phys. Chem. A., 106, 11130 (2002)
[2] Y. B. Alfassi; W. A. Prutz; R. H. Schuler: J. Phys. Chem., 90, 1198 (1986)
P49
Physical-chemical characterization of historical wooden samples consolidated by radiation polymerization
I. Stanculescu, Department of Physical Chemistry, Faculty of Chemistry, University of Bucharest, 4-12 Bd. Regina Elisabeta, 030018 Bucharest, Romania
M. Virgolici, M. M. Manea, R. Suvaila, M. Cutrubinis, D. C. Negut, C. C. Ponta, IRASM Irradiation Technology Center, .Horia Hulubei. National Institute for Physics and Nuclear Engineering,
407 Atomistilor Str., 077125, Magurele, Ilfov County, Romania
Wooden samples older than 300 years, collected from three Romanian churches were studied. The consolidation and disinfection was done by impregnation and radiation polymerization of a styrene unsaturated polyester tetrahydrophtalic resin. Bruker Vertex 70 class FT-IR spectrometer equipped with a fiber optic mobile Raman probe (RAMPROBE) attached to Raman module (RAM II, LN2 cooled Ge detector) was used for non-distructive in situ Raman measurements at the wood surface and FT-IR spectra acquisition using KBr pellets. TGA-IR external unit accessory for Vertex 70 FT-IR spectrometer coupled with NETZSCH STA 409 PC Luxx simultaneous thermal analyzer was used for structural characterization of evolved gases from samples during thermal analysis. FT-IR spectra of wood samples (KBr method) showed differences in band intensity and position for various wood types. The relative change in the ratio lignin (1508 cm-1)/carbohydrate (1375 cm-1) reference bands was used to assess the biological decay. In the FT-Raman spectra the polymerization process was evidenced by the presence of new bands at 1734 cm-1 and 1002 cm-1 and various changes in intensity and position of other peaks. Before consolidation treatment thermal analysis revealed water content between 2 and 5 % and no significant differences were found in the FT-IR spectra of main pyrolysis products evolved under inert atmosphere from all types of wood.
Vibrational spectra analysis provided information about changes in the molecular structure of different wood type components due to biological or photophysical decay and in situ polymerization by irradiation. Also, thermal analysis coupled with FTIR spectroscopy has good potential in the investigation of chemical composition of wooden cultural heritage objects.
P50
New methods in modelling recombination kinetics and spin dynamics
A. Agarwal, N. Green, Department of Chemistry, University of Oxford, UK
There are two main methods for modelling recombination and spin dynamics in radiation chemical systems: a Monte Carlo random flights method (MC), in which the trajectories of the diffusing species are followed explicitly; the Independent Reaction Times method (IRT), in which reaction times are sampled from appropriate marginal distribution functions. This paper reports recent developments to both methods, which extend the range of systems for which they can be used.
The main chemical problem with the IRT method is the problem of dealing with secondary reactions of reactive products. The main difficulty is the requirement to generate distances from the new particle to the remaining reactants. Several methods have been proposed in the past, none of which is wholly satisfactory. This paper reports a new .First Passage Approach. algorithm that allows the new distance to be generated conditional on the IRT that already existed for that pair. This removes a source of bias that is present in the current methods. Tests against full MC simulations show (i) that the new method introduces new particles with the correct spatial distribution and (ii) that it is more accurate than current methods in modelling the subsequent kinetics.
Methods for simulating coherent spin evolution in radiation chemical systems have been proposed and implemented before, but it has not been possible to include incoherent spin relaxation in the Hilbert space formalism used. A method for simulating spin relaxation based on discrete events suggested by Brocklehurst has been implemented in both MC and IRT models and tested for a single radical pair against methods using Liouville space and the density matrix formulation. Essentially exact agreement is found, permitting spin models to be generalised with the addition of spin relaxation (both T1 andT2).
Spin effects leading to polarisation require the action of distance-dependent interactions such as exchange. It is straightforward to include these in trajectory based MC simulations as the spin evolution becomes an integration along the diffusion path. However, it has always been thought to be impossible to do this using the much faster IRT method. By partitioning the space surrounding each particle into small shells and including the first passage times between these shells it is possible to generalise the IRT method to model these interactions with a comparable accuracy to the MC method, both for neutral and charged pairs. This new method makes it possible to simulate effects such as polarisations with a much better statistical precision than before because of the ability to perform many more realisations.