Structure studies on micro- and mesoporous ferri- and stannisilicates
Background
Micro- and mesoporus substances may play role in varous applications, e.g. in catalytic processes. They can be considered as analogous structures to the natural minerals, i.e. to zeolites. Zeolites are mostly alumino-silicates with various pore structures (pore sizes of ca. 0.5 - 1.0 nm). A number of these natural structures and even more, artifical ones never existing in the nature, can be synthesized in pure siliceous forms. The pure siliceous framework is chemically neutral, its properties can be modified by inserting small amounts of other elements - e.g. iron or tin into the framework. These two elements are easily accessible for Mössbauer spectroscopic studies as well. This method is particularly advantageous for identifying the oxidation and coordination states, thus it is very useful for structure studies. These kind of studies have been carried out in our Department since a while. To illustrate the variety of the systems and their possible changes in catalytic processes, six examples are presented. Namely,
1. process of the solid state ion exchange in the NH4-Y + FeCl2 => Fe-Y + NH3 + HCl,2. identification the Lewis and Bronsted sites, their participation in alkylation reactions in Fe-MFI,
3. simultaneous presence of framework Fe3+ and extra-framework Fe2+ in the N2O decomposition under reaction conditions at 620 K,
4. characterisation of composite Fe-SBA-15 substances, their application in total oxidation of phenol,
5. stannisilicates: the extent of reversibility for Sn4+ <=> Sn2+ in micro- and mesoporous hosts,
are briefly described in the following.
1. Solid state ion exchange in the NH4-Y + FeCl2 => Fe-Y + NH3 + HCl process
Introduction of modifying ions into the zeolite framework usually is a process consisting of several steps, e.g. ion exchange from solution, or pore impregnation etc. Virtually a simple mixing (grounding in a mortar) of the solid components might also be considered as a possible procedure. And, surprisingly, it may ven work - under special conditions - namely when the original charge compensating counterion may be simply removed - e.g. by heating. This possibility has been demonstrated in the NH4Y + FeCl2 => Fe-Y + NH3 + HCl process, as shown by the temperature programmed mass spectrometry and in situ Mössbauer measurements. The selected host was Y (faujasite type) zeolite, providing large "super"-cages and cubooctahedral sodalite units for the components (Fig. 1.1).
 |
|
First the so-called NH4-form of the Y-zeolite is prepared - where the charge compensation against the framework Al3+ ions is provided by NH4+ cations. In the next step these charge compensating cations can be replaced by Fe2+ in the NH4Y + FeCl2 => Fe-Y + NH3 + HCl process. The procedure is simple: the appropriate amount of the Y zeolite is mixedwith FeCl2 . 4 H2O then the mixture is grinded in a mortar, and , afterwards, heated in vacuum up to 570 K. The process can be followed by mass spectrometry of the removed components (see Fig. 1.2)
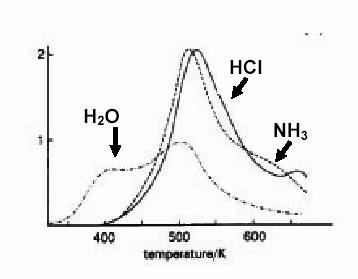 | Fig . 1.2. Temperature programmed decomposition of the FeCl2 + NH4-Y mixture as followed by mass spectrometry . It is clearly seen that the main transformation, the removal of NH4Cl proceeds at 500 - 550 K via decomposition to NH3 and HCl |
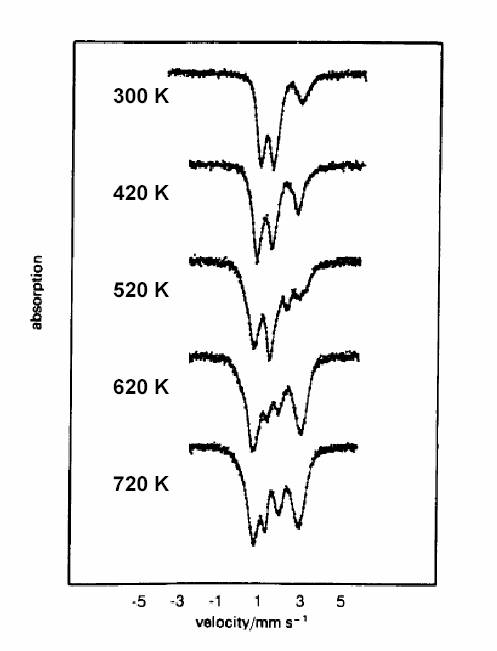 | Fig 1.3. Mössbauer spectra recorded on the FeCl2 . 4H2O + NH4-Y imixture after treatments at various temperatures in vacuum. |
These spectra can be decomposed to the constituent doublets of ferrous and ferric ions of different coordinations. Specifically, eight different coordinations can be distinguished. Their appearances as the relative contributions in percentages are shown in Fig. 1.4.
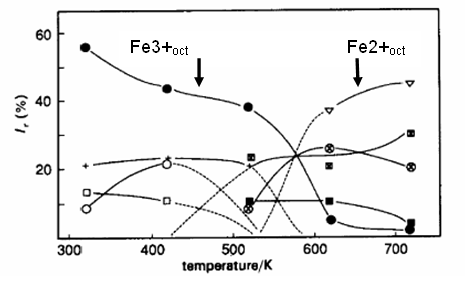 | Fig. 1.4. Contributions of components of different coordinations identified in the Mössbauer spectra of Fig. 1.3. independence of the temperature of treatments |
As seen from the figure, the most significant transformations in the coordination and oxidation states of the various iron species proceed in the 500 - 550K region. The main feature is the conversion of the predominantFe3+oct componentto Fe2+oct upon the raise of the temperature. Most probably theoriginal FeCl(OH). H2O entities are present as larger neutral associations in the supercages at the start, and in correspondence, iron can be detected exhibitingoctahedral coordination characteristic for the mentioned moieities. As the temperature surpasses the critical 500 - 550 K, several processes take place simultaneously. The most important ones are:
- NH4 is removed from the extra-framework charge compensating sites in form of NH3,
- the remaining acidity (H+, or H3O+) is also removed in form of HCl,
- most of the Fe3+ is reduced to Fe2+ (probably by the NH3 evolved),
- Fe2+ migrates into the tighter cubooctahedrons, since the octahedral coordination is retained, however in a coordination which is cleary different from that characteristic for the starting octahedral one in the FeCl2. 4H2O.
2. Identifying the Lewis and Bronsted sites, and their participation in alkylation reactions on Fe-MFI
Iron
ions may be located not only at the extra-framework sites (e.g.
as was shown in the previous case of solid-state ion exchange), but
they may replace the framework building silicon ions as well. In
this latter case, similarly to the case of the more frequent
substitution with Al, Bronsted acidic sites are generated with
removable protons from the (Si-O)3-Fe(OH)-Si groups.
On the charge-compesating extra-framework sites, wihout the removable
proton, Lewis acidic centres may be formed.
One of the favoured zeolite structures is the ZSM-5 (or MFI) - it is stable and is used in various reactions (Fig. 2.1).
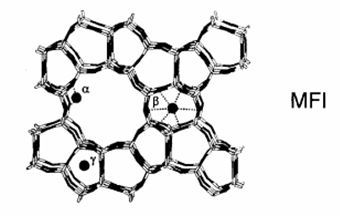 | Fig. 2.1. The structure of MFI . The
large 10 member ring channels have a diameter of c.a 0.5 nm
providing tight place for reactions. Among the extra-framework sites
mostly the ions located in "alpha" position may
participate, the "beta" and "gamma" sites inside the pores are mostly inaccessible for the various reactions. |
To obtain information on the participation of the various types of iron ions (framework substituted and extra-framework, i.e. possessing Bronsted or Lewis acidities, respectively) disproportionation of toluene and alkylation of toluene with ethylene were studied on Fe-MFI with different iron contents (with Si/Fe ratios 27.5, 35 and 67). The different types of iron were distinguished by in situ Mössbauer spectroscopy, their portions were correlated to acitivities, shown in the conversion reactions of toluene. The portions of the framework and extra-framework types of iron were determined from Mössbauer spectra recorded after a treatment in hydrogen at 620 K - the framework ferric ions usually are not reduced at this temperature, whereas the ferricions located at extra-framework sites cn be reduced to ferrous form at 620 K in hydrogen. The component marked asFe(III) Td is considered to originate from the framework substituted iron, since the (Si-O)3-Fe(OH)-Si environment is strongly asymmetric, as indicated by the detected large large quadrupole splitting ( QS > 1.2 mm/s) on the Fe(III) component. All the Fe(II) components are attributed to extra-framework species, since they were reduced (Fe3+ =>Fe2+) with the hydrogen treatment.
| Fig. 2.2.In situ spectra on Fe / MFI | r = Si/Fe | Comp | IS | QS | RI | |
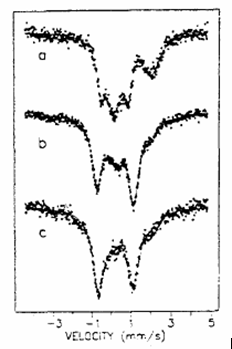 recorded after 620 K / H2 treatment | 27.5 (a) | Fe(III) Td Fe(II) Td Fe(II) Oh Fe(II) Oh | 0.15 0.56 1.01 1.19 | 1.24 0.70 1.76 2.25 | 44 9 26 21 | |
| 35 (b) | Fe(III) Td Fe(II) Td Fe(II) Oh | 0.25 0.66 1.03 | 1.92 0.66 2.10 | 59 20 21 | ||
| 67.0 (c) | Fe(III) Td Fe(II) Oh | 0.23 1.13 | 1.76 2.06 | 85 15 |
The dependences of the initial conversion rates in reactions of toluene on the iron contents and the estimated amounts of framework substituted (FW) and extra-framework (EFW) iron are shown in Fig. 2.3. It is shown that the influence of the FW iron is more expressed on the reaction rate than that of the EFW type,since the strong increase of the amount of the latter type results only in a modest raise of the overall activity. Thus it can be concluded that iron located in FW sites is more favoured for these type of reactions.
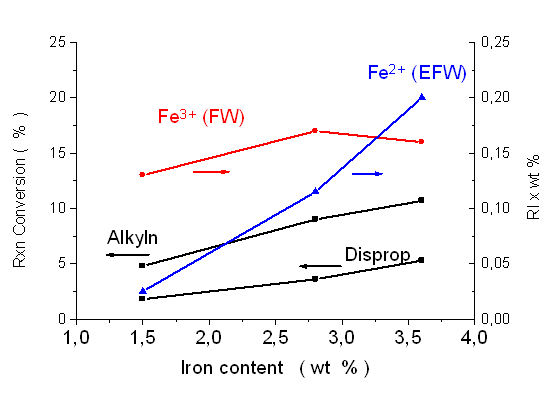 | Fig. 2.3. Comparisons of toluene conversion rates in dependences of the amounts of framework (FW) and extra-framework (EFW) types of iron |
K. Lázár, A. M.-Szeleczky, G. Vorbeck, R. Fricke, A. Vondrova, J. Cejka: J. Radioanal. Nucl. Chem., 190 (1995) 407 - 411, and
K. Lázár, R. Fricke, H. Kosslick, J. Cejka, G. Vorbeck, A.M.-Szeleczky, Stud. Surf. Sci., Catal., 94 (1995) 219 - 225.
3. Simultaneous presence of framework Fe3+ and extra-framework Fe2+ in N2O decomposition under reaction conditions at 620 K
In the previous chapter it was shownthat Fe3+ => Fe2+ reduction may proceed on extraframework ions (in H2 at 620 K), and this feature was utilised to distinguish between FW and EFW ions. Thus, completion of the opposite process, the Fe2+ => Fe3+ oxidation at 620 K in strongly oxidizing media (e.g. in N2O), could obviously be expected. In the recent chapteran important exemption is presented - stabilization of EFW Fe2+ ions can be demonstrated under reaction conditions of decomposition of N2O. This stabilization is related to an imporatant reaction, namely, to the generation of particularly active, so-called "alpha" oxygen, which can be used e.g. to oxidise benzene to phenol at room temperature. The key component in this process is a ferrisilicate (in most cases Fe-MFI) activated in N2O. In close connection, Fe-MFI is also particularly active in decomposition of N2O, in our case Fe-MFI was investigated in the latter process.
A particular Fe-MFI sample was synthesized for this study. In the first stage FW iron ions were introduced by hydrothermal synthesis using enriched 57Fe isotope (specifically sensitive to the Mössbauer effect), in Si / 57Fe = 200 ratio. The synthesis was successfull: Mössbauer spectra recorded on this sample exhibited presence of separated single Fe3+ ions. Due to their low concentration, paramagnetic relaxation was observed asdisplayed in the broad wide lines shown in the spectra of on the left side of Fig. 3.1.
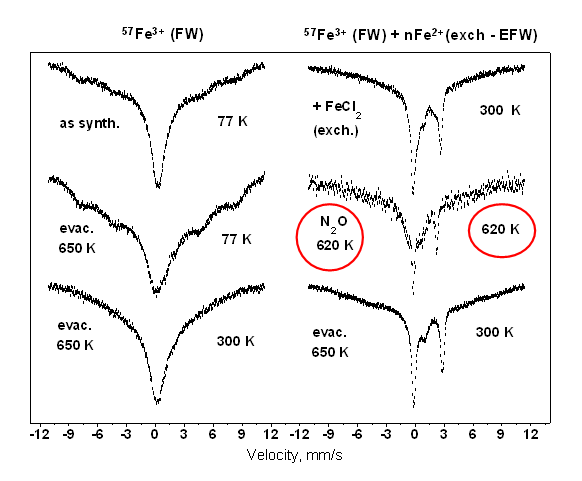
Fig. 3.1. in situ Mössbauer spectra of the Fe-MFI (the treatments are shown below the spectra - left, and the temperature of measurements - right). After hydrothermal synthesis of 57Fe/Si ~ 200 (left side): Fe3+ is located in FW sites. In a subsequent stage EFW Fe2+ was introduced by ion exchange ( right side). Note the stabilisation of ferrious iron under reaction conditions of decomposition of N2O at 620 K (middle).
In the next stage EFW iron was introduced with ion exchangefrom methanolic solution of FeSO4 (with regular iron, i.e. containing the 57Fe
isotope only in 2.7 %). From the determination
oftotalamount of iron a Si/Fe ~ 50 ratio was
estimated,corresponding to ca. FeEFW / FeFW ~ 3. Thus, EFW is the major type of the ironspecies present.
In correspondence, thedoublet of EFW Fe2+ is superimposed to the broad component originated from theparamagnetic FW Fe3+on the spectrumrecorded after ion exchange (Fig. 3.1. top, right). A spectrum was collected in the next step, during the decomposition of N2O at 620 K. Surprisingly, the doublet of EFW Fe2+ is still clearly displayed (Fig. 3.2. middle, right). At the end, a spectrum was recorded after an evacuation - the Fe2+ was still present. Thus, the series of spectra shown in Fig. 3.1 clearly demonstrates that EFW Fe2+ exists under the strongly oxidising conditions in N2O atmosphere.
In a separate experiment the catalalytic activity of the sample
from the same batch of preparation was measured in a temperature
programmed decomposition measurement, by determining the activity as
the amount of decomposed N2O (Fig. 3.2). The
highest activity was detected in the range of 600 - 620 K, proving
clearly that the Fe-MFI catalyst is active.
Thus, combining the
results of the TPR and Mössbauer data it can be suggested that the
active component in the decomposition of N2O is of EFW ferrous type.
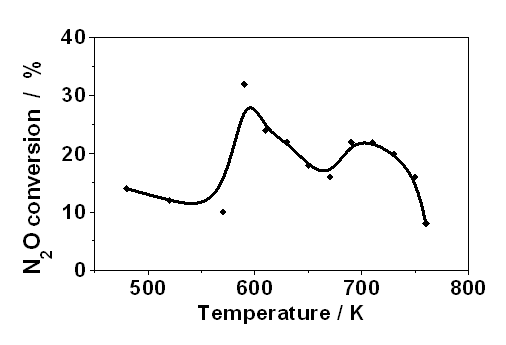 | Fig. 3.2. Rate of N2O decompostion on Fe-MFI measured by Temperature Programmed Decomposition (10 K / min) |
Further details of this study are reported in K. Lázár, O. Pozdnyakova, A. Wootsch, P. Fejes, Hyperfine Interactions, 167 (2006) 779 - 784.
4. Characterization ofcomposite Fe-SBA-15 substances, their application intotal oxidation of phenol
The
SBA-15 belongs to the family of the mesoporous structures. They
consist of wide pore (3 - 6 nm in diameter) channels, and the structure
of the wall in between the channels is mostly amorphous as is
demostrated withcharacteristic XRD of these substances exhibiting
characteristic reflection peaksonlyat in the 5 deg < (2
theta) region.
The large pore opening is an advantageous property of
thesesubstances, e.g. they can accommodate large molecules when
using them in catalytic processes.
 | Fig. 4.1. Schematic structure of mesoporous substances. They are composed from orderedchannels of c.a. 3 - 6 nm in diameterand are separated with partly amorphous pore walls with thickness of 1 - 2 nm |
| Sample | pH | Fe cont (%) |
| DS-1 | <1 | 1.3 |
| DS-2 | 3.5 | 16 |
| DS-3 | 7 | 22 |
The transmission electron micrographs provedthe homogeneous mesoporous structure for the DS-1 sample is and parallel EDX studies revealed that iron is uniformly distributed in this substance. The mesoporous structure was also dominant in DS-2 and DS-3 samples, however, large iron-rich regions were also present in them.
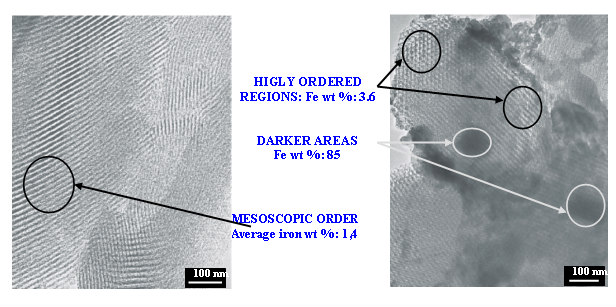
Fig. 4.2. TEM and EDX on DS-1 (left side) and DS-2 (right side) samples
Mössbauer spectra were also obtained on the three samples (Fig. 4.3.)
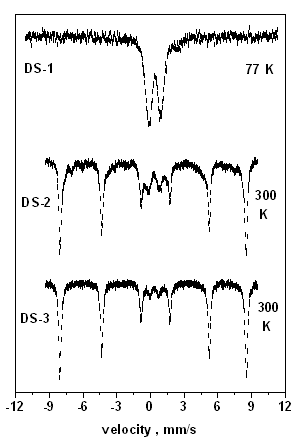 | Fig . 4.3. Mössbauer spectra of Fe-SBA-15 samples. The spectrum of DS-1 sample (top) is characteristic for iron incorporated in ionic dispersion to the siliceous matrix, whereas the main component in the spectra of DS-2 and DS-3 samples is hematite - appearing in the form of the magnetically split sextet(77 and 300 K are the temperatures of measurements). |
Curiously, the three Fe-SBA-15 substances, in spite of their significantly different iron content exhibit similar activities when used as catalysts for the total oxidation of phenol in aqueous media with H2O2 at 100 oC under 7 bar pressure (Fig. 4.4).
In a subsequent stage the three samples were evaluated in the catalytic process of total oxidation of phenol performed in aqeous phase at 100 oC under 7 bar pressure of air with hydrogen peroxide as oxidizing agent. The activity was determined as proportional to the removed part of phenol analysed insamples taken periodically from the reaction mixture.
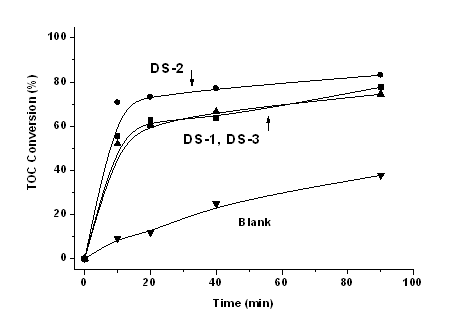 | Fig. 4.4. Activities of the Fe-SBA-15 samples in total oxidation of phenol in aqueous media with H2O2 at 100 oC |
Further details of this study and comparison with Fe-SBA-15 samples of other type are reported in K. Lázár, G. Calleja, J.A. Melero, F. Martinez and R. Molina,Stud. Surf. Sci. Catal., 154 (2004) 805 - 812.
5. Stannisilicates: Sn4+ <=> Sn2+ reversibility in micro- and mesoporuos hosts
Tin
can also be introduced into micro- and mesoporousstructures
forming various stannisilicates. Redox changes on tin ions (Sn4+ <=> Sn2+)
are two-electron processes, i.e. they can be related to removal or
uptake of one oxygen atom. Thus, this change can be utilized in
one-step redox processes. The extent of reversibility and
stabilisation of Sn2+ may depend on the actual structure. In our studies the extents of Sn4+ <=> Sn2+ reversible process were
compared on various microporous (MFI, MEL, MTW) and mesoporous (SBA-15
and MCM-41) stannisilicates. The treatment was the same:
reduction in hydrogen at 670 K than reoxidation in air at 520 K. The
substances were evaluated as catalysts in liquid phase oxidation
processes.
To illustrate the properties of microporous
silicates spectra recorded on Sn-MFI are shown in Fig. 5.1.
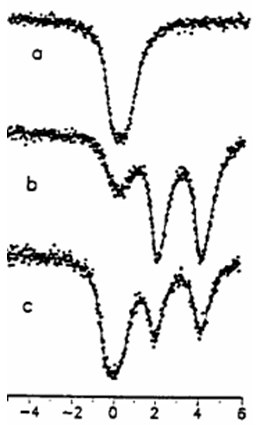 | Fig. 5.1. In situ Mössbauer spectra of Sn-MFI sample, recorded at 77 K. - synthesized and calcined (a), - treated in hydrogen at 670 K (b), - reoxidised in air at 520 K (c) Spectrum (a) exhibits the singlet of Sn4+. Upon reduction a doublet of Sn2+ develops - the two lines with same intensityon right side of (b), with a minor part of the original Sn4+ (b- left) . Reoxidation results only in partial restoration of Sn4+. |
The SBA-15 mesoporous structure is synthesized under rather acidic conditions, thus, the pore walls are partially ordered so that they are crystalline in an extent. In other words, the portion of free silanolic groups in the pore walls is notpredominant.
Corresponding in situ Mössbauer spectra recorded under the same conditions as applied previously are shown in Fig. 5.2.
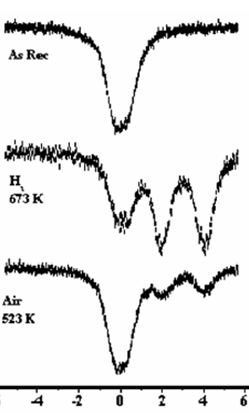 | Fig 5.2. In situ Mössbauer spectra recorded at 77 K on Sn-SBA-15 |
Another type of mesoporous stannisilicate, Sn-MCM-41 was also synthesized. The synthesis proceeds under less acidic conditions than used for SBA, thus the pore walls are thinner and may contain silanolic groups in larger extents as shown in the scheme (Fig. 5.3).
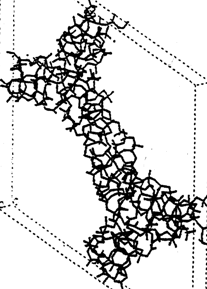 | Fig. 5.3.The mostly amorphous structure and the large number of the silanolic groups is illustrated on the scheme of walls of the mesoporous MCM-41 structure. |
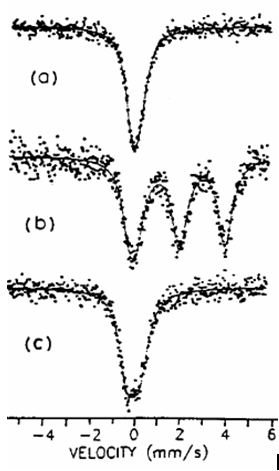 | Fig. 5.4. In situ Mössbauer spectra recorded on Sn-MCM-41 - as synthesized and calcined (a), - treated in H2 at 670 K (b), - reoxidized in air at 520 K (c) |
Thus, the important role of the structure on the stabilization of various species, and thereby on the redox processes are demonstrated.
The Sn-MCM-41 was also tested in catalytic processes, in hydroxylation of phenol and naphtol, as well as epoxidation of norbornene with tertiary butyl hydroperoxide. The catalytic performance was reasonable (~ 50 % conversion). Two points are worth mentioning here:
- the size of reactant molecules are certainly large, in particular in the latter example, these processes could not be catalysed with microporous catalysts,
- the use of the solid phase Sn-ferrisilicates as catalysts in the processes provide a mean for easy separation of catalysts from the products, otherwise homogeneous-phase catalysts should be used, resulting in the use of more troublesome separation processes.
- K. Lázár, A.M. Szeleczky, N.K. Mal, A.V. Ramaswamy, Zeolites, 19 (1997) 123 - 127,
-
on mesoporous SBA-15 and MCM-41 in
- P. Shah, A.V. Ramaswamy, K. Lazar, Veda Ramaswamy, Microporous and Mesoporous Materials, 100 (2007) 210 - 226, and
- K. Chaudhari, T.K. Das, P.R. Rajmohanan, K. Lazar, S. Sivasanker, A.J. Chandwadkar, Journal of Catalysis, 183 (1999) 281 - 291, respectively.

